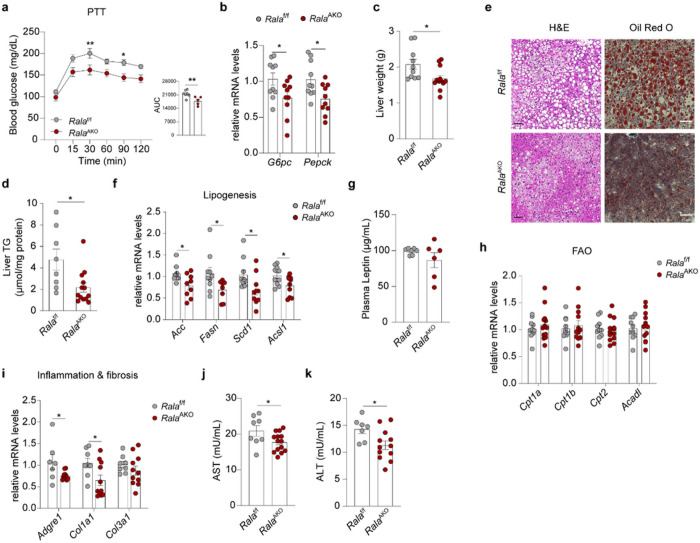
Figure 2. Loss of RalA in WAT ameliorates HFD-induced hepatic steatosis.
a, Pyruvate tolerance test (PTT) was performed on overnight fasted Ralaf/f and RalaAKO mice (n = 5–7) after 8 weeks of HFD feeding. AUC was calculated from PTT longitudinal chart, b, Relative mRNA expression of key gluconeogenic genes in livers of HFD-fed Ralaf/f and RalaAKO mice (n = 10). c, Liver weight of HFD-fed Ralaf/f and RalaAKO mice (n = 10–12). d, Triglyceride (TG) content in livers of HFD-fed Ralaf/f and RalaAKO mice (n = 8–13). e, Representative H&E staining (left; n = 3) and Oil-Red-O staining (right; n = 3) of liver sections in HFD-fed Ralaf/f and RalaAKO mice, scale bar =15 mm. f, Relative mRNA expression of lipogenic genes in livers of HFD-fed Ralaf/f and RalaAKO mice (n = 9–10). g, Plasma leptin levels in HFD-fed Ralaf/f and RalaAKO mice (n = 6–7). h, Relative mRNA expression of fatty acid oxidation (FAO) related genes in livers of HFD-fed Ralaf/f and RalaAKO mice (n = 10–11). i, Relative mRNA expression of genes related to inflammation and fibrosis in livers of HFD-fed Ralaf/f and RalaAKO mice (n = 7–11). j, k, Plasma AST (j) and ALT (k) activities in HFD-fed Ralaf/f and RalaAKO mice (n = 7–14). The data (a-d, f-k) are shown as the mean ± SEM, *p < 0.05, **p < 0.01 by unpaired t-test (b-d, f-k) or two-way ANOVA with Bonferroni’s multiple comparison as post-test (a).