Abstract
An 88-year-old woman underwent atrioventricular node ablation and left bundle branch pacing for atrial fibrillation. She presented to the emergency room several hours after discharge with dyspnea. An echocardiogram revealed a giant interventricular septal hematoma. The patient was successfully treated with conservative medical therapy, with eventual complete resolution of the hematoma. (Level of Difficulty: Intermediate.)
Key Words: complication, conduction system pacing, echocardiography, interventricular septal hematoma, left bundle branch pacing
Central Illustration
An 88-year-old woman was referred for atrioventricular (AV) node ablation and pacemaker implantation with conduction system pacing because of atrial fibrillation (AF) refractory to antiarrhythmic therapy with amiodarone and intolerant to AV nodal blocking drugs because of hypotension. His bundle pacing (HBP) lead was attempted but abandoned because of high capture thresholds (2 V at 1 ms). Subsequently, a left bundle branch pacing (LBBP) lead (Medtronic 3830) was implanted in the proximal left bundle branch (LBB) location (V6 R-wave peak time 70 ms, QRS duration 135 ms, LBB–ventricle interval 27 ms). After AV node ablation from axillary vein access, a second lead was implanted at a slightly distal LBB location (V6 R-wave peak time 61 ms, QRS duration 121 ms) with low capture thresholds (0.5 V at 0.5 ms). Six hours after same-day discharge, the patient presented to the hospital with nausea, vomiting, and dyspnea. Her vital signs were within normal limits (BP 130/80 mm Hg; heart rate 82 beats/min, temperature 98.6 °F, oxygen saturation 97%). On examination, she was noted to be ill-appearing, with dry mucous membranes, left pectoral pacemaker site without ecchymosis or hematoma, and mild epigastric tenderness. Otherwise, the result of the clinical examination was unremarkable.
Learning Objectives
-
•
To describe a rare complication of a giant interventricular septal hematoma following left bundle branch pacing.
-
•
To discuss management of interventricular septal hematoma.
-
•
To recognize unforeseen complications in a relatively novel treatment strategy of physiological pacing.
Medical History
The patient’s history was significant for persistent AF with rapid ventricular response. She had previously undergone 3 cardioversions on amiodarone therapy. She had a history of coronary artery disease, peripheral artery disease with percutaneous intervention and stent placement, remote cerebrovascular accident, renal insufficiency, and hiatal hernia. Her discharge medications included clopidogrel, rivaroxaban, atenolol, chlorthalidone, and amlodipine.
Differential Diagnosis
The differential diagnoses for the patient’s presenting nausea, vomiting, and dyspnea were concerning for, but not limited to, gastrointestinal, cardiovascular (acute coronary syndrome, heart failure, anemia), neurologic (hemorrhagic vs ischemic cerebrovascular accident), and postprocedural (pericarditis, pericardial tamponade or periprocedural medication intolerance) conditions.
Investigations
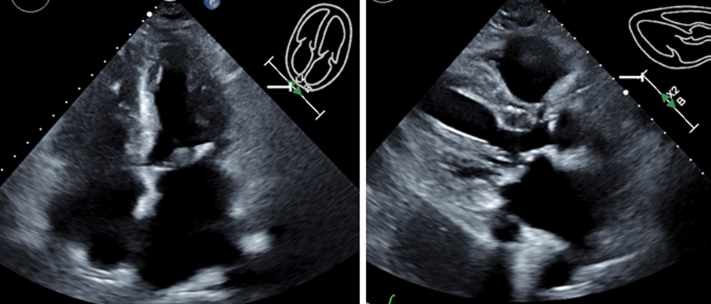
The admission workup noted hemoglobin 16.3 g/dL and platelets 193,000/μL. An electrocardiogram and a chest X-ray were unremarkable (Figure 1). Laboratory workup noted elevated cardiac markers (high-sensitivity troponin T 4,217 ng/L, creatine kinase-myocardial band 273 ng/mL, creatine kinase 1,002 U/L, B-type natriuretic peptide 17,221 pg/mL), and increase in serum creatinine from 1.8 to 2.2 mg/dL. An urgent echocardiogram revealed a large septal hypoechoic mass (61 mm × 40 mm) with near-complete obliteration of the right ventricle (RV), no pericardial effusion, and otherwise normal left ventricular systolic function (Figure 2, Videos 1 and 2). There was no significant respiratory variation in mitral inflow, and approximately 20% tricuspid inflow variability. Computed tomography of the chest without contrast material noted cardiomegaly and an equivocal hyperdense mass in the RV. Pacemaker parameters including pacing thresholds and impedances were unchanged from implantation.
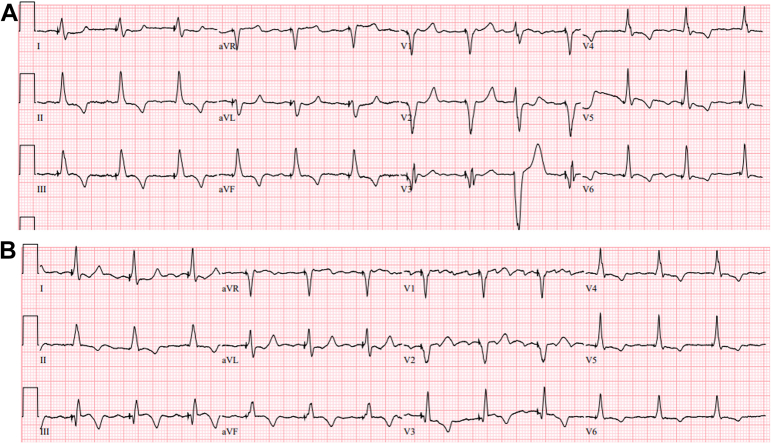
Figure 1.
Paced Electrocardiograms
(A) Immediately after left bundle branch pacing lead implantation. (B) On presentation at Emergency Department.
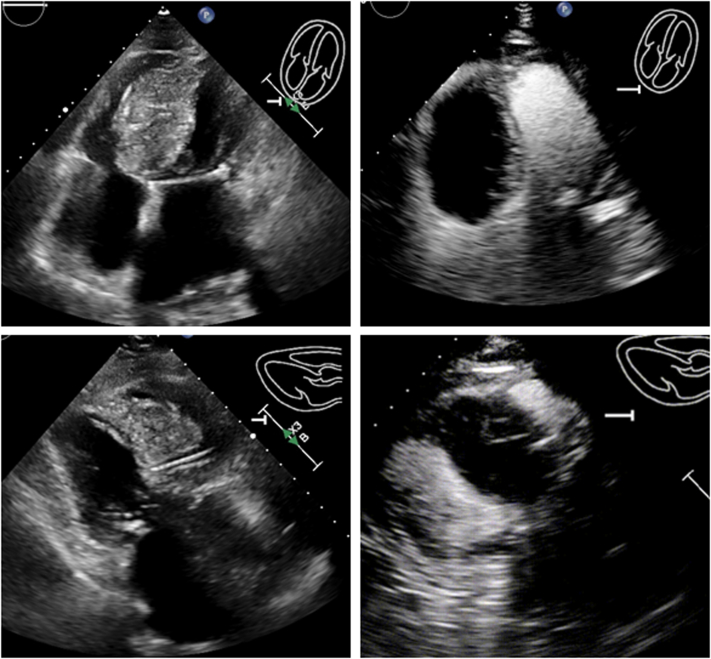
Figure 2.
Presenting Echocardiograms Postoperative Day 1
Large septal hematoma measuring 61 mm × 40 mm is visible in apical 4-chamber and parasternal long-axis views with and without contrast material.
Management
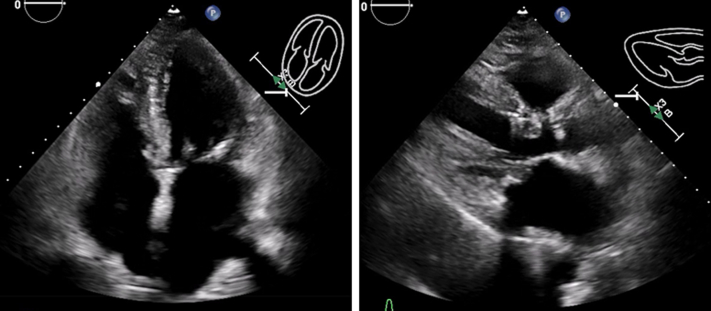
Initially, rivaroxaban and clopidogrel were held, and intravenous fluids were administered. Given the recent LBBP, the significant elevation in cardiac enzymes, new echocardiographic images, and CT findings were consistent with the development of a large interventricular septal (IVS) hematoma, likely caused by bleeding from septal perforator arteries in the setting of uninterrupted anticoagulation and antiplatelet therapy. After a multidisciplinary discussion between critical care, interventional cardiology, and electrophysiology, a decision was made to manage conservatively. The patient’s symptoms progressively improved and remained hemodynamically stable during hospitalization. Serial echocardiograms during hospitalization noted improved RV filling and stable hematoma size without progression to pericardial effusion. The patient was subsequently discharged on day 7 in stable condition. An echocardiogram at 2 weeks showed a decrease in the hematoma, and oral anticoagulation was resumed (Figure 3). A repeated echocardiogram at 6 weeks showed complete resolution of the hematoma, and the pacemaker parameters continued to remain stable with persistent LBB capture (Figure 4, Videos 3 and 4).
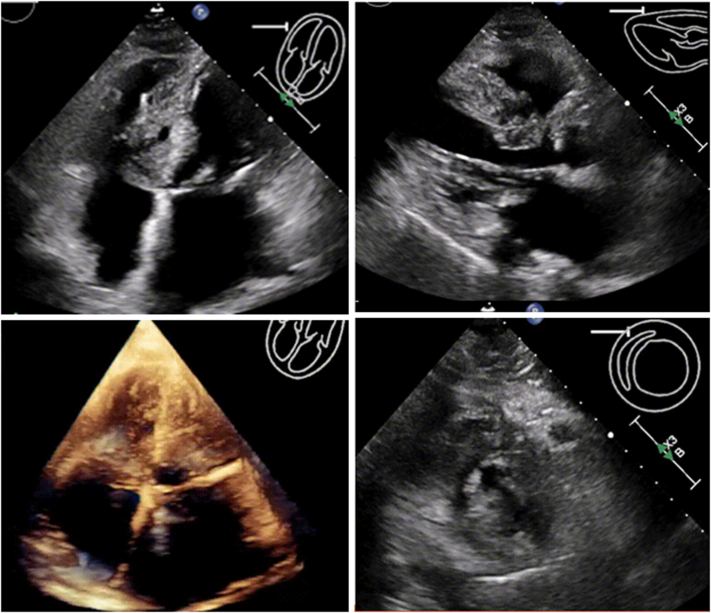
Figure 3.
Follow-Up Echocardiograms at 2 Weeks
Interval improvement in septal hematoma size and improvement in right ventricle size are visible in apical 4-chamber and parasternal long-axis views.
Figure 4.
Follow-Up Echocardiograms 6 Weeks Postoperatively
Complete resolution of hematoma and normal biventricular size and systolic function are visible.
Discussion
LBBP has been associated with improved clinical outcomes compared with RV pacing.1 AV node ablation and LBBP are increasingly used to achieve rate control in patients with AF.2 This new modality of physiological pacing may be associated with unusual complications.3 This is a rare case of a giant IVS hematoma successfully managed conservatively. This is also the first case of IVS hematoma in our center out of >2,000 cases of physiological pacing lead implantation. The recent cardiac procedure, atypical abdominal symptoms, and significantly elevated cardiac enzymes (out of proportion to the cardiac procedure) despite no significant electrocardiographic findings of ischemia led to urgent echocardiographic evaluation and early diagnosis of this rare complication.4 The size and extent of septal hematoma protruding into the RV and the rapidity of occurrence was a concern about progressive hemodynamic compromise caused by potential RV inflow restriction/obstruction or extension of hematoma into the pericardial space leading to tamponade.
Large septal perforator arteries are present in the proximal anterior IVS. Injury to these vessels was the likely cause of our patient’s presenting intraseptal hematoma. Given the therapeutic oral anticoagulation and antiplatelet therapy, it is likely that the hematoma expanded rapidly. Wu et al5 recently showed the successful use of coil embolization for bleeding septal perforator resulting in septal hematoma after septal radiofrequency ablation. Successful coil embolization in a patient with septal hematoma after LBBP complicated by pericardial tamponade was recently reported.6 Coronary angiography to localize the bleeding septal perforator and possible coil embolization was discussed but was not performed because of our patient’s renal dysfunction and hemodynamic stability. Alternatively, removal of the LBBP leads in the hope of creating a path to drain into the RV was considered but not pursued because of the unpredictability of this approach, possible clot formation at the exit site, and potential inability to place a new pacing lead in the restricted RV. Puncturing the RV septum at its thinnest region close to the hematoma under echocardiographic guidance to evacuate the hematoma was also considered. After discussion with the heart team, the patient, and her family, a decision was made to initially manage conservatively with close hemodynamic and echocardiographic follow-up, which ultimately was effective in this patient.
Follow-Up
The patient remained clinically asymptomatic during outpatient re-evaluation at 2 weeks postoperatively. A repeated echocardiogram noted resolving hematoma with normal RV filling, no pericardial effusion, and normal biventricular systolic function. Subsequently, anticoagulation and antiplatelets were resumed. A repeated echocardiogram at 6 weeks and 3 months postoperatively showed complete resolution of the IVS hematoma (Figure 5). Of note, both LBBP lead thresholds were noted to be stable throughout the clinical course.
Figure 5.
Follow-Up Echocardiograms 3 Months Postoperatively
Resolved hematoma and stable biventricular size and function are visible.
LBBP is a novel technique. Whereas septal hematoma is a rare complication, lead implantation in the high anteroseptal or low posteroseptal region should be avoided where large perforator arterial branches are present. Additionally, careful management of periprocedural anticoagulation and antiplatelet therapy may be necessary to avoid this unusual complication.
Conclusions
We describe an unusual case of giant interventricular septal hematoma complicating pacemaker implantation with LBBP leads that was successfully managed without invasive procedural intervention.
Funding Support and Author Disclosures
Dr. Vijayaraman has been a consultant for Abbott, Biotronik and Eaglepoint LLC; holder of a patent for HBP delivery tool and the recipient of honoraria and research and fellowship support from Medtronic; a consultant for Abbott and Biotronik; and the holder of a patent HBP delivery tool for Eaglepoint LLC. All other have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
Admission 4-Chamber Echocardiographic View of Septal Hematoma Measuring 61 mm × 40 mm
Admission 4-Chamber Echocardiographic View With Contrast Material of Septal Hematoma
6-Week Follow-Up Echocardiogram (Apical 4-Chamber View) Showing Complete Resolution of Hematoma
6-Week Follow-Up Echocardiogram (Parasternal Long-Axis View)
References
- 1.Sharma P.S., Patel N.R., Ravi V., et al. Clinical outcomes of left bundle branch area pacing compared to right ventricular pacing: results from the Geisinger-Rush conduction system pacing registry. Heart Rhythm. 2022;19(1):3–11. doi: 10.1016/j.hrthm.2021.08.033. [DOI] [PubMed] [Google Scholar]
- 2.Vijayaraman P., Mathew A.J., Naperkowski A., et al. Conduction system pacing versus conventional pacing in patients undergoing atrioventricular node ablation: nonrandomized, on-treatment comparison. Heart Rhythm O2. 2022;3:368–376. doi: 10.1016/j.hroo.2022.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jastrzębski M., Kiełbasa G., Cano O., et al. Left bundle branch area pacing outcomes: the multicentre European MELOS study. Eur Heart J. 2022;43(40):4161–4173. doi: 10.1093/eurheartj/ehac445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ponnusamy S.S., Patel N.R., Naperkowski A., Subzposh F.A., Vijayaraman P. Cardiac troponin release following left bundle branch pacing. J Cardiovasc Electrophysiol. 2021;32:851–855. doi: 10.1111/jce.14905. [DOI] [PubMed] [Google Scholar]
- 5.Wu B., Zhou Y., Lu J., et al. Interventricular septal hematoma caused by percutaneous intramyocardial septal radiofrequency ablation successfully treated with coil embolization. J Am Coll Caardiol Intv. 2023;16(6):722–724. doi: 10.1016/j.jcin.2022.10.038. [DOI] [PubMed] [Google Scholar]
- 6.Chen X., Lu H., Xu L., et al. Interventricular septal hematoma with pericardium effusion after left bundle branch pacing implantation. J Am Coll Cardiiol EP. 2023;9:142–144. doi: 10.1016/j.jacep.2022.09.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Admission 4-Chamber Echocardiographic View of Septal Hematoma Measuring 61 mm × 40 mm
Admission 4-Chamber Echocardiographic View With Contrast Material of Septal Hematoma
6-Week Follow-Up Echocardiogram (Apical 4-Chamber View) Showing Complete Resolution of Hematoma
6-Week Follow-Up Echocardiogram (Parasternal Long-Axis View)