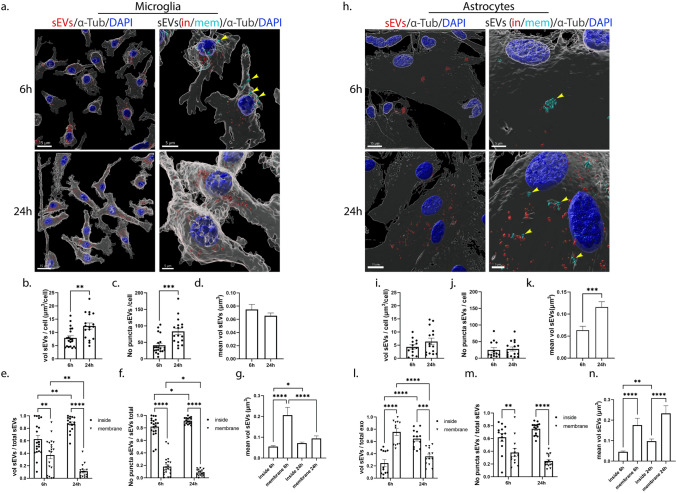
Fig. 2.
Brain-derived sEVs are internalized in primary microglia and astrocytes. Primary cells were incubated with Dil-stained brain-derived sEVs (depicted in red) for 6 h and 24 h. Cells were washed, and internalization of sEVs was monitored 6 h and 24 h post-incubation. Cells were fixed and immunostained with an antibody against α-Tubulin (α-Tub) (gray), while cell nuclei were stained with DAPI (blue). Confocal images were deconvolved and analyzed with the Imaris Imaging software. sEVs were defined as cytoplasmic (sEVs in, red) or membranous (sEVs mem, cyan blue) using the ‘’Distance transformation’’ module of the Imaris Imaging software, computing the distance of the sEV puncta from the ‘’α-Tubulin’’ surface. Representative Imaris images depict the internalization of sEVs masked with the α-Tubulin surface (left panel, scale bar 15 μm) and in/mem sEVs (right panel, scale bar 5 μm) in microglia (a) and astrocytes (h), 6 h and 24 h post-addition. ‘’sEVs mem’’ were depicted with arrowheads. Graphs show the total volume of internalized sEVs per cell (b, i), the number of puncta per cell (c, j), the mean volume of sEVs (d, k), the ratio of the volume of sEVs (in/mem) per total volume (e, l), the ratio of the number of puncta (in/mem) per total number (f, m), and the mean volume of sEVs (in/mem) (g, n), in microglia and astrocytes, respectively. Data are presented as the mean ± SEM of minimum three independent cell preparations, with at least two replicates per assay; Student's t test was used for (b), (d), (i), and (k), Mann–Whitney test for (c) and (j), one-way ANOVA with Tukey’s correction for (g) and (n), two-way ANOVA with Tukey’s correction for (e) and (m), and multiple t test for (f) and (m). Statistical significance was set as *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001