Figure 4: Nanoscale arrangement and rearrangement of dyadic calcium channels.
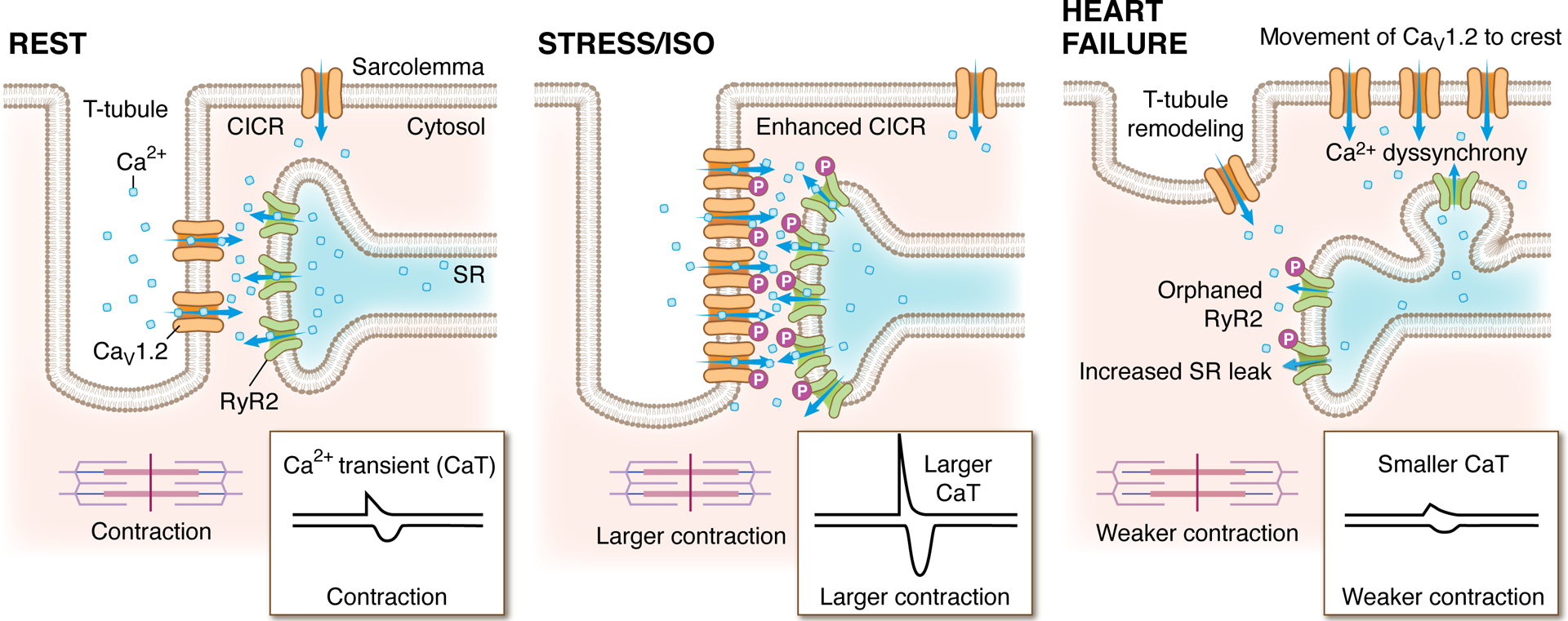
The localization and degree of clustering of cardiac CaV1.2 and RyR2 channels influences functional output. These cartoon depictions of the cardiac dyad, illustrate the clustering state and localization of CaV1.2 channels and juxtaposed RyR2 in resting (left), acutely stressed (middle), and heart failure conditions (right). At rest, clusters of CaV1.2 channels are located on the t-tubules opposite clusters of RyR2 on the jSR. During exercise, acute stress, or isoprenaline (ISO) application, the ensuing β-AR/Gs/adenylyl cyclase/cAMP/PKA signaling cascade results in phosphorylation of both CaV1.2 channels and RyR2, increasing their activity (Po). In parallel, PKA stimulates enhanced recycling of CaV1.2 from an endosomal reservoir to augment CaV1.2 number and cluster size at the t-tubules. On the jSR, RyR2 clusters also undergo remodeling, becoming enlarged during stress. Altogether, the combination of enhanced channel Po, increased levels of cooperative gating, and larger numbers of CaV1.2 channels within enlarged clusters leads tο greater Ca2+ entry which stimulates more CICR. Larger RyR2 clusters also generate larger, more frequent sparks which summate to form larger Ca2+ transients and stronger contractions, creating the characteristic positive inotropic response during acute stress. Right: In heart failure, cardiomyocytes undergo architectural remodeling and ion channel cluster rearrangements. RyR2 channel cluster fragmentation/dispersal and orphaning occur as jSR elements are left behind while t-tubules recede. CaV1.2 channels migrate to the crest but the reduced proximity to RyR2 leads to an inefficient CICR that results in Ca2+ dyssynchrony. Hyperphosphorylation of RyR2 increases their activity leading to SR leak which elevates diastolic Ca levels in the cytosol and reduces the releasable pool in the SR that can be accessed during CICR. The result is smaller Ca2+ transients and weaker contractions.
