Abstract
Cancer is the second leading cause of death worldwide despite efforts in early diagnosis of the disease and advances in treatment. The use of drugs that exert toxic effects on tumor cells or chemotherapy is one of the most widely used treatments against cancer. However, its low toxic selectivity affects both healthy cells and cancer cells. It has been reported that chemotherapeutic drugs may generate neurotoxicity that induces deleterious effects of chemotherapy in the central nervous system. In this sense, patients report decreased cognitive abilities, such as memory, learning, and some executive functions after chemotherapy. This chemotherapy-induced cognitive impairment (CICI) develops during treatment and persists even after chemotherapy. Here we present a review of the literature on the main neurobiological mechanisms involved in CICI using a Boolean formula following the steps of the PRISMA guidelines that were used to perform statements searches in various databases. The main mechanisms described in the literature to explain CRCI include direct and indirect mechanisms that induce neurotoxicity by chemotherapeutic agents. Therefore, this review provides a general understanding of the neurobiological mechanisms of CICI and the possible therapeutic targets to prevent it..
Key words: Chemobrain; Cognitive impairment, Neurotoxicity; Chemotherapy drugs, CICI
Graphical Abstract
Highlights
-
•
Cancer treatment can cause chemotherapy-induced cognitive impairment (CICI).
-
•
CICI or chemobrain is the general concept to explain cognitive impairments.
-
•
Cognitive deficits have been reported in patients and cancer survivors.
-
•
CICI’s mechanisms include neurotoxicity, inflammation, and impaired neurogenesis.
1. Introduction
Cancer is one of the leading causes of death worldwide [47]. In 2020, the World Health Organization reported 19.292.789 new cancer cases, with breast cancer being the most prevalent type [87]. Hallmarks of these polygenic diseases are replicative immortality, angiogenesis induction, resisting cell death, sustaining proliferative signaling, evading growth suppressors, activating invasion and metastasis, cellular energetics dysregulation, genome instability, and mutation, eluding immune destruction, and tumor-promoting inflammation [40]. Due to cancer’s etiology complexity, each patient requires different types of treatment or a combination of them, chemotherapy and radiotherapy are among the main treatments, given their effectiveness in increasing patient survival with different types of cancer [86].
Several chemotherapeutic drugs are used in cancer treatment, classified by the mechanism of action. Alkylating agents (cyclophosphamide, cisplatin, carboplatin) and nitrosoureas are DNA crosslinkers [22]. Anti-metabolites such as fluorouracil and methotrexate inhibit DNA synthesis, and plant alkaloids (vincristine, paclitaxel, docetaxel) induce apoptosis through DNA damage [38]. Anti-tumor antibiotics inhibits topoisomerase II [21], or inhibits the synthesis and repair mechanisms of DNA [17]. Nevertheless, an essential issue of chemotherapy drugs is their effects on healthy and cancer cells, inducing multiple side effects.
Epidemiological studies in the United States record that as of 2019, there were 16.9 million cancer survivors [7]. It has been reported that one side effect of chemotherapy for the treatment of peripherical tumors and central nervous system tumors is known as “chemobrain,” “chemo fog,” or cancer-related cognitive impairments. Chemobrain includes impairment of learning, memory, attention, and executive function impairment [49], [74]. Other studies have described that the prevalence of cognitive impairment after chemotherapy is estimated to be 15 %− 75 %, and up to 17 %− 35 % suffer long-term effects [61], [63].
Common symptoms reported by patients suffering from CRCI include the inability to solve problems, lack of concentration, difficulty finding the right words when speaking, memory loss, learning disabilities, impaired executive functions, and processing speed [61], [69]; other neurobehavioral changes affecting the patient's quality of life after chemotherapy are anxiety and depression [57]. Even when it has been reported that just having a cancer diagnosis can affect cognitive function, evidence supports that chemotherapy further alters proper cognitive functioning [48].
Epidemiological studies can be expected to report an increasing number of new cancer cases in the coming years and consequently the number of reports of patients with cognitive impairment will increase. Currently, research is focused on understanding the neurobiological mechanisms underlying CICI and the search for therapeutic targets [64], [77]. Although some mechanisms underlying CICI have been described, knowledge about the effects of different chemotherapeutics on cognition is constantly growing. This review aims to present a comprehensible update on the leading neurobiological mechanisms involved in chemobrain.
2. Methodology
PRISMA guidelines [58] were used to perform a scoping review to identify the literature update the leading neurobiological mechanisms involved in chemotherapy-induced cognitive impairment (CICI). First, several databases were selected from the electronic sources provided by the Pontificia Universidad Javeriana (Academic Search Complete, Google Scholar, Nature, ProQuest, ScienceDirect, Scopus, SpringerLink, Taylor & Francis, and Web of Science). Then, the Boolean search was performed with the following formula: chemobrain AND mechanism AND biochemical AND cellular. Inclusion criteria were defined as follows: original articles or reviews in English, peer-reviewed, published in the period 2012–2021, with clinical, in vivo, and in vitro study models. Additional articles were identified from the reference lists of previously selected articles and chosen for further review; in this case, the publication date was not considered.
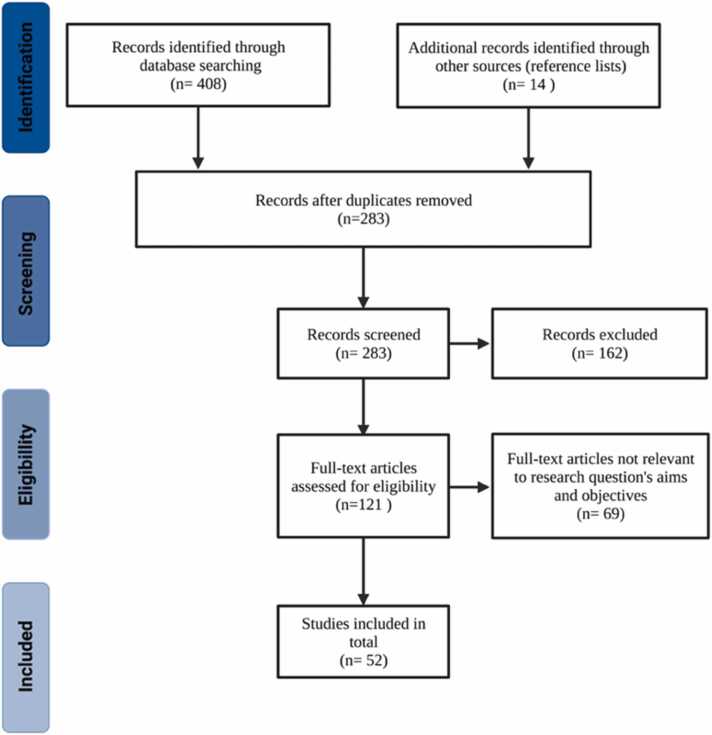
PRISMA flowchart depicts the articles and reviews' selection process (Fig. 1). All previously obtained articles were reviewed, and duplicates were excluded. Other exclusion criteria were as follows: articles that corresponded to abstracts or conference reports, articles on neurodegenerative diseases, computational approaches, neurobehavioral changes, or articles in which the chemotherapy regimen was not specified. Subsequently, the selected articles were read and analyzed, and the information was summarized in Table 1. Finally, the results section briefly describes each neurobiological mechanism in the table.
Fig. 1.
Preferred Reporting Items for Systematic Reviews (PRISMA) flowchart for articles and reviews search process. 408 documents were identified with the Boolean search in the following databases: Academic Search Complete = 39, Google Scholar = 157, Nature = 2, ProQuest = 50, ScienceDirect = 22, Scopus = 122, SpringerLink = 12, Taylor & Francis, = 3 and Web of Science = 1. Additional documents from the reference lists = 11. Finally, 283 records were screened and finally 51 articles were selected for full-text review. Created with BioRender.com.
Table 1.
Neurobiological mechanisms involved in the development of Cancer-related cognitive impairment.
| Type of chemotherapy drug | Mechanism of action | Chemotherapy agent | Neurobiological mechanisms |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Neurotoxicity | Diminish neurogenesis | Loss of dendritic spines and arborizations | Alterations in neurotransmission | Disturbance in glial cells | Myelination loss | Cytokine dysregulation | |||
| Alkylating agents | DNA crosslinker[22] | Cyclophosphamide | [75],[43],[54] | [26] Reviewed in[43] | [32] | [26] | [44] | ||
| Cisplatin | [28],[22],[25] | [77],[10] Reviewed in[43] | [67],[10] | [12] | [77] | [45],[12] | |||
| Carboplatin | [66] | [49] | |||||||
| Nitrosoureas | DNA crosslinker[22] | Carmustine | [59] | Reviewed in[43] | |||||
| Anti-metabolites | Inhibits DNA synthesis[22] | Fluorauracil | [14] | [14],[43] | [37] | [46] | [39] | [39] | [37] |
| Methotrexate | [22],[66],[59] | [85] | [2] | [34],[35] | [34],[35] | ||||
| Plant alkaloids | DNA damage inducing apoptosis[38] | Vincristine | [59],[43],[60] | ||||||
| Paclitaxel | [42],[56],[66],[78] | [42] | [56] | ||||||
| Docetaxel | [30],[66],[78] | [30] | |||||||
| Anti-tumor antibiotics | Inhibits topoisomerase II[21], or inhibits the synthesis and repair of DNA[17] | Doxorubicin | [22],[73],[82] | [26] | [83],[32] | [4],[11],[79] | [20] | [5],[78],[82] | |
| Mitoxantrone | [6] | ||||||||
| Combination | - | BEP (bleomycin, etoposide, cisplatin) | [8] | ||||||
| CMF (cyclophosphamide, methotrexate, and 5-fluororacil) | [18] | [9] | [19] | [19] | |||||
| DAC (Docetaxel, Doxorubicin, Cyclophosphamide) | [74] | [74] | |||||||
| AC (Doxorrubicin, and cyclophosphamide) | [13] | [13] | |||||||
3. Results
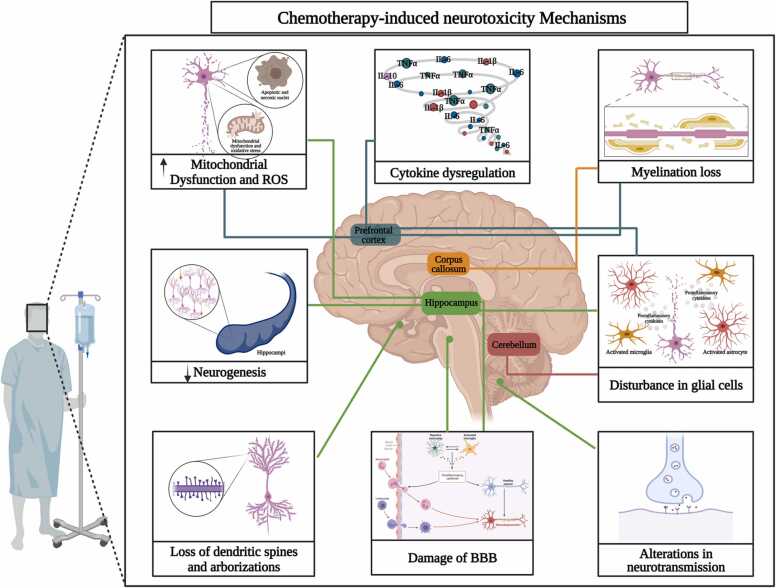
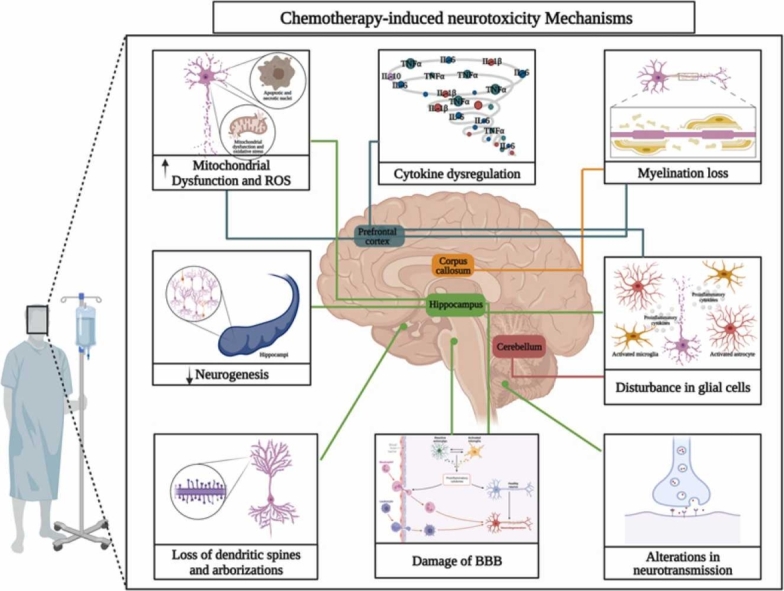
This section will be briefly described the main neurobiological mechanisms underlying CRCI found in the literature search (Table 1), including neurotoxicity, decreased neurogenesis, loss of dendritic spines and dendritic complexity, altered neurotransmission, disruption of glial cells, loss of myelination and cytokine dysregulation leading to neuroinflammation (Fig. 2).
Fig. 2.
Chemotherapy-induced Neurotoxicity Mechanisms. The blue lines indicate mechanisms occurring in the prefrontal cortex, the green lines indicate mechanisms occurring in the hippocampus, the orange line indicates processes occurring in the corpus callosum and the red line indicates processes occurring in the cerebellum. Created with BioRender.com.
3.1. Mechanisms of neurotoxicity
Neurotoxicity is related to damage, dysfunction, and neurodegeneration of the neurons in the Central Nervous System (CNS) or the peripheral nervous system (PNS) [66]. The functionality of the CNS depends on the interaction between neurons and glial cells (astrocytes, oligodendrocytes, and microglia). Exogenous agents such as chemotherapeutic drugs have been reported to be toxic to the nervous system and other organs such as bone marrow and lungs, so chemotherapeutics doses are limited [66].
Chemotherapy-induced neurotoxicity may affect neurons through mechanisms that decrease functionality directly or indirectly. Neurotoxicity is a crucial mechanism underlying CICI as it directly impairs the homeostasis and viability of neurons in essential brain areas involved in cognitive processes. Some neurotoxic effects rely on increased oxidative stress, mitochondrial dysfunction, and apoptosis. Consequently, the loss of neuronal plasticity induces a decrease in neurogenesis, decreased dendritic arborization, loss of spines and demyelination, and alterations related to neurotransmission that may cause neurodegeneration. In addition, damage indirectly caused by the effect of glial cells (macroglia or microglia), such as f neuroinflammation and damage to the BBB, are highlighted. This section will review the direct and indirect mechanisms of chemotherapy-induced neurotoxicity.
3.1.1. Chemotherapeutics induce direct mechanisms of neurotoxicity
3.1.1.1. Mitochondrial dysfunction and oxidative stress
Neurotoxicity induced by chemotherapeutic agents has also been linked to mitochondrial dysfunction, oxidative stress, and consequent DNA damage. Platinum derivatives such as oxaliplatin and cisplatin may enter neuronal cells and binding to mitochondrial DNA (mDNA), forming mDNA adducts that cannot be repaired in mitochondria due to the absence of DNA repair systems. These platinum-mDNA adducts disrupt physiological mDNA replication and transcription, which can lead to abnormal protein synthesis and impairment of the function of the mitochondrial electron transport chain, in turn, to decreased cellular metabolism, increased production of ROS (reactive oxygen species), and oxidative stress [88]. In addition, it has also been reported that cisplatin induces mitochondrial dysfunction as evidenced by decreased respiratory capacity and morphological alterations, events related to the poor performance of treated animals in new object/place recognition (NOPRT) tests [25]. Likewise, the cisplatin analog, carboplatin, produces mitochondrial damage and oxidative stress ([24] reviewed in [66]). A study in C57/BL6J mice treated with cisplatin (34.5 mg/kg cumulative dose) impaired performance on the novel object and place recognition task, as well as on the social discrimination task, suggesting that treatment with cisplatin-induced cognitive deficits that are associated with structural abnormalities in the brain relative to induced morphological abnormalities in the brain on the level of white matter organization, neuronal arborization, and dendritic spine density [89]. In this same model of cisplatin-induced cognitive impairment, all have been observed peripheral neuropathy was observed with neuroprotection from metformin treatment, which was associated with protection against mitochondrial damage and decreased neuroinflammation [89]. Another study showed elevated oxidative stress (nitrotyrosine, 4-hydroxynonenal) and DNA damage (pH2AX, pATM) in cortical neurons from chemotherapy patients. The study identifies markers of oxidative stress and DNA damage in autopsy tissue sections of the frontal lobe of cancer patients treated with chemotherapy (n = 15), cancer patients not treated with chemotherapy (n = 10), and patients with no history of cancer (n = 10). Of the known chemotherapy regimens, the most frequently administered drugs were cisplatin (42.9 %), carboplatin (28.6 %), cyclophosphamide (28.6 %), doxorubicin (28.6 %), pemetrexed (28.6 %), %), paclitaxel (28.6 %), cytarabine (21.4 %). %), etoposide (21.4 %), vincristine (21.4 %) and docetaxel (14.3 %), gemcitabine (14.3 %), ifosfamide (14.3 %), azacitidine (7.1 %), decitabine (7.1 %), fluorouracil (7.1 %), irinotecan (7.1 %), eribulin (7.1 %), busulfan (7.1 %), fludarabine (7.1 %), daunorubicin (7.1 %) and oxaliplatin (7.1 %). Patients received a median of 3 chemotherapeutic agents (range 2–8) and were treated with a median of 5.5 cycles of chemotherapeutic regimens (range 1–17). This work shows for the first time that cancer patients treated with chemotherapy have elevated markers of oxidative stress and DNA damage in cortical neurons compared with cancer patients not treated with chemotherapy and patients without a cancer history. Importantly, these observations highlight the relevance of human neuronal oxidative stress and DNA damage in studying the mechanisms that may contribute to CRCI [80].
Chemotherapeutic agent doxorubicin (DOX) has been implicated in cognitive decline, mostly via cytokine-induced neuroinflammatory and oxidative and mitochondrial damage to brain tissues. Cognitive dysfunction was induced in female Wistar rats by administering ten cycles of DOX (2.5 mg/kg, intra-peritoneal, once in 5 days), as we observed significant impairment of episodic memory in object recognition task (ORT). Also, DOX human neuroblastoma (IMR32) cells in vitro study resulted in increased cellular death, apoptosis, and intracellular ROS generation, with inhibition of neurite growth in differentiated IMR32 cells [68]. On the other hands, DOX-induced neurotoxicity Wistar male rat model led to neurobehavioral alterations and neurochemical deficits in the Hippocampus region. This study depicted the persistent increase in proinflammatory cytokines (IL-1β, TNF-α) that led to increased oxidative stress, lipid peroxidation, depletion in GSH level, and changes in vivo antioxidant defense SOD and CAT activity. This study depicted the persistent increase in proinflammatory cytokines (IL-1β, TNF-α) that led to increased oxidative stress, lipid peroxidation, depletion in GSH level, and changes in vivo antioxidant defense SOD and CAT activity. The authors showed that the animals with DOX significantly impaired mitochondrial redox activity, decreasing the mitochondrial respiratory chain complex I, II, and IV. In addition, the results have shown increased oxidative stress markers such as MDA level, reduction in GSH level, and reduction activities of SOD and CAT in the hippocampus of animals DOX treated group [33].
Another study with Male C57BL/6 mice DOX-induced chemobrain model intraperitoneal injections of 2.5 mg/kg DOX every two days for a total of seven injections over a 2-week period. The results showed elevated pro-inflammatory levels (TNF-α, IL-1β, and IL-6) and a marked increase in Iba1 and GFAP immunoreactivity in the brains of DOX-treated animals. In addition, systemic DOX administration triggered oxidative damage as indicated by a significant elevation in the levels of MDA, protein carbonyl, and 8-OHdG, depletion of reduced GSH, and reduction of SOD activity. DOX impaired brain mitochondrial function, as evidenced by the decreased mitochondrial respiratory complex activities, the ATP deficiency, and the increased ROS in DOX-treated mice. The results also demonstrated that decreased synaptic density and dendritic spine loss were paralleled with mitochondrial dysfunction after DOX exposure [83]. The above results suggest that chemobrain is a potential side effect of doxorubicin administration and the key role of cytokine-induced, oxidative/nitrosative stress in the development of cognitive dysfunction. Doxorubicin-induced neurotoxicity was also associated with acute alterations in synaptic plasticity, apoptosis, and lipid peroxidation, suggesting an increase of oxidative stress in a hippocampal cell line (H19–7/IGF-IR) [3].
3.1.1.2. Loss of neuronal plasticity mechanisms
3.1.1.2.1. Reduced neuronal survival
It has been described in a murine model that intraperitoneal injections of cyclophosphamide (CPA) cause histopathological damage in the cerebral cortex, characterized by necrotic and apoptotic nuclei [75]. Similarly, intraperitoneal injections of paclitaxel in a murine model increased apoptosis in hippocampal neurons by increasing proinflammatory cytokines [56]. Furthermore, reduced neuronal survival has also been reported using cisplatin in an in vitro model of cortical neurons [28]. In this case, astrocyte-mediated mitochondrial transfer induced recovery of cisplatin-treated neurons. In this case, astrocyte-mediated mitochondrial transfer induced recovery of cisplatin-treated neurons. On the other hand, it was reported that acute cisplatin treatment causes an increase in apoptotic cells in the CA1, CA3, and subgranular zone (SGZ) of the dentate gyrus in the hippocampus of male rats [10]. Also, it has been reported that DOX induced histopathological damage showing nuclear pyknosis in mice hippocampal neurons, particularly in the subiculum, fascia dentata, and hilus, along with darker cytoplasm and abnormal morphology [73]. Interestingly, an in vitro assay differentiated SH-SY5Y human neuronal cell model, mitoxantrone was more toxic than DOX, with decreased viability and the observation of apoptotic nuclei. Further assays revealed that cytotoxicity was mediated by depolarization of the mitochondrial membrane potential after 48 h of exposure to chemotherapeutics [6]. Other studies have shown that another chemotherapeutic drug, vincristine, destroys hippocampal tissue when administered unilaterally or bilaterally to mice [60].
3.1.1.2.2. Diminishing neurogenesis
Neurogenesis is a complex process in which a wide range of cellular and molecular events generate fully mature postmitotic neurons. In the adult mammal brain, neurogenesis has been documented in different regions, including the olfactory bulb, neocortex, striatum, and hippocampus [76]. Notably, particular emphasis has been given to hippocampal neurogenesis due to its relationship with higher cognitive functions, especially with learning and memory [52]. In this vein, it has been reported in a murine model that intraperitoneal injections of cyclophosphamide and doxorubicin caused a 47 % and 53 % decrease in the number of immature doublecortin (DCX)-labeled neurons, respectively when compared to saline treated controls; also, ectopic migration of these immature neurons was observed [26]. A decrease in neurons labeled simultaneously with NeuN (a marker for mature neurons) and BrdU (a marker for proliferative cells) was also observed after treatment with these chemotherapeutics, suggesting that hippocampal neurogenesis was significantly impaired [26], [43]. Similarly, the use of the CMF (cyclophosphamide, methotrexate, and fluorouracil) regimen in an in vivo model also resulted in a decrease of proliferative cells in the hippocampus, specifically in the dentate gyrus [18].
Brain-Derived Neurotrophic Factor (BDNF) plays a critical role in the growth, development, plasticity, differentiation, and survival of new neurons, and reduction in its expression leads to memory deficits. It has been reported that the systemic administration of fluorouracil induces a decrease of DCX and BDNF-positive cells in the hippocampus ([62] reviewed in [14]), therefore suggesting a disruption in hippocampal neurogenesis (Reviewed in [43]). Moreover, the systemic administration of fluorouracil reduced the expression of the BDNF levels in rat hippocampus, affecting learning and memory ([62] reviewed in [14]).
Other study reported that cisplatin treatment resulted in the depletion of neural stem cells (NSCs) cultured in vitro; however, NSCs were more resilient than differentiated hippocampal neurons [10]. Notably, antioxidant treatments can prevent the deleterious effect of cisplatin and carmustine on hippocampal neurogenesis, suggesting that oxidative stress is an important mechanism in this context (Reviewed in [43]; Reviewed in [77]).
Comparable whit these results, it was reported that intravenous injection of methotrexate in rats significantly reduced DCX expression in the hippocampus. This effect was accompanied by a decrease in BDNF and Nuclear factor erythroid-related factor (Nrf2) levels in both the hippocampus and prefrontal cortex, both factors critical in the regulation of neurogenesis and subsequent differentiation of NSCs [50], [85]. The decline in NSCs viability has also been observed in paclitaxel-treated cells in culture [42].
As evidenced above, several chemotherapeutics decrease the expression of neurotrophic factors allowing impaired neurogenesis in critical areas for learning and memory. It is suggested that impaired NSCs production, migration, and differentiation is a possible neurobiological mechanism underlying chemotherapeutic-induced cognitive impairment.
3.1.1.2.3. Loss of dendritic arborizations and spines
Neuronal connectivity and function are highly related to the number of dendritic spines and dendritic tree complexity [10]. Dendritic spines are specialized protrusions in the excitatory synapses [23]. These tiny structures have been reported as critical in synaptic plasticity because their morphology and density are essential for memory and learning processes [23]. Four types of dendritic spines have been proposed based on their morphology: mushroom spines, thin spines, stubby spines, and cup-shaped spines, while mushroom spines are considered the most stable thin spines are considered the least stable [23]. The potential importance of dendritic spines in memory and learning has been described from long-term potentiation (LTP). In this process, the spines with the highest activity will be maintained over time [51]. Similarly, it has also been documented that the complexity of the dendritic tree has an essential role in memory formation and consolidation [2].
Several chemotherapeutics can cause a reduction in the density of dendritic spines and dendritic branches. Intravenous injection of cyclophosphamide or doxorubicin in ovariectomized mice reduced the density of stubby dendritic spines in the dentate gyrus [32]. Similarly, intraperitoneal injection of doxorubicin in mice decreases the density of dendritic spines and synaptic density in neurons of the CA1 region of the hippocampus [83]. Also, in vivo and in vitro models, it was documented that treatment with cisplatin caused a reduction in the density of dendritic spines and dendritic branches in apical and basal dendrites in pyramidal neurons of the CA1 and CA3 regions of the hippocampus [10]. The synaptic damage induced by cisplatin was not reversible, even after five days post-treatment [10].
Similar results show that the intrathecal application of methotrexate using a juvenile murine model considerably affects the dendritic architecture and reduces the density of mushroom dendritic spines in CA1, CA3, and dentate gyrus of the hippocampus [2]. Consistent with these results, it was reported in another murine model that the intraperitoneal application of docetaxel, doxorubicin, and cyclophosphamide (DAC) significantly eliminated dendritic spines of medial prefrontal cortex neurons [74].
More complex effects were reported in CMF-treated animals that exhibited a significant increase in the number of stubby spines and a decrease in the number of mushroom-shaped spines in the DG, while in CA1 and CA3 regions of the hippocampus, there was a significant increase in the density of dendritic spines [9]. However, decreases in the number of branch points, dendritic ends, dendritic length, and dendritic complexity were observed in CMF-treated animals. In addition, it has been described in an aged murine model that the use of intraperitoneal injections of fluorouracil caused a decrease in the density of mushroom dendritic spines in the DG while the density of thin and stubby dendritic spines increased. Accordingly, apical dendrites of pyramidal neurons of the CA1 region showed a decreased density of mushroom spines but an increased density of stubby spines. However, a decrease in thin spines was documented in the basal dendrites of pyramidal neurons in CA3 [37]. Also, it has been that fluorouracil negatively affected dendritic complexity in DG neurons [37].
These results indicate that chemotherapeutic treatment affects dendritic spine dynamics and that there is also a tendency to decrease dendritic complexity in pyramidal neurons of the hippocampus. One of the most marked trends was the decreased number of mushroom dendritic spines and the increase in thin and stubby spines. Given that mushroom spines are the most stable and the thin spines the least stable, changes in the density ratio of these spines could explain, in part, the development of chemobrain.
3.1.1.2.4. Alterations in neurotransmission
Neuronal communication occurs via an electrochemical gradient; the action potential travels through the neuron cell body and generates neurotransmitter release to the synaptic cleft [29]. It has been seen that the most important brain regions in cognition, such as the prefrontal cortex and the hippocampus, are densely innervated with serotonergic, cholinergic, and dopaminergic afferents. Therefore, neurotransmitters such as serotonin, acetylcholine, and dopamine (DA) are essential modulators of memory and learning [36] [55]. Also, glutamate plays a vital role in cognition, specifically in spatial memory, given that hippocampal neurons are glutamatergic [41].
It has been described an increased brain acetylcholine levels in a Rat Model of Cisplatin-Induced Cerebral Inflammation and Oxidative Damage while generating a decrease in the relative expression levels and activity of acetylcholinesterase [12]. In contrast, in another in vivo model, it has been reported that the use of doxorubicin produces a reduction in the levels of acetylcholine in both hippocampus and prefrontal cortex [4]. Researchers found that DOX treatment impaired learning and memory [4]. Also, decreased dopamine release in the striatum was documented after intravenous application of fluorouracil in a murine model; these findings were correlated with the decrease in attention shown by these animals [46]. Similar results were obtained after treating animals with carboplatin; DA and serotonin release were negatively affected while DA uptake was decreased in the striatum; these changes that correlate to the impairment in spatial learning exhibited by the carboplatin-treated animals [49]. Additionally, it has been found that intraperitoneal application of doxorubicin generates a decrease of DA and its extra-neuronal metabolite 3-methoxytyramine (3-MT) in both the frontal cortex and hippocampus of treated animals compared to the control [11]. In parallel, another research group documented that doxorubicin produced a slower glutamate uptake in the frontal cortex, while the dentate gyrus produced a slower clearance of glutamate and, consequently, an overflow of glutamate in this region [79]. All this together affected the swimming speed of the doxorubicin-treated group; however, this effect lasted only 24 h after administration [79].
As mentioned before, chemotherapeutic agents can affect the homeostasis of different neurotransmitters suggesting that the release or capture of neurotransmitters can be negatively compromised and thus contribute to the deterioration of cognitive processes associated with memory and learning, common symptoms presented by patients with CRCI.
3.1.1.2.5. Demyelinating processes in brain
In the CNS, myelination is a complex and lifelong process in which oligodendrocytes wrap segments of the axons of specific neurons. These segments become isolated and enable the saltatory action potential, which is an evolutionary mechanism that allows faster conduction and processing of information. ([1]; de [31]). Two types of myelination have been described. The first is intrinsic myelination, which occurs independently of neuronal activity. The second is adaptive myelination, which is regulated and shaped by neuronal activity and is associated with neuroplasticity, which is critical for learning [16]. The deleterious effect of chemotherapeutics on myelinization has been reported in a murine model; methotrexate exposure has been documented to decrease the cortical expression of BDNF, which, together with TrkB, is required for adaptive myelination [34]. Interestingly, BDNF expression is normalized after microglia depletion, although the underlying mechanisms are unknown [34], [35]. Also, toxic effects on CNS progenitor cells and non-dividing oligodendrocytes have been reported with the use of fluorouracil both in vivo and in vitro [39]. Administration of fluorouracil generates delayed damage in myelin and axons, which can be observed up to 56 days after the completion of the treatment, resulting in a myelopathy that did not correlate with chronic inflammation or vascular damage caused by the chemotherapeutic [39]. Similarly, it has been documented that the use of a CMF regimen generates a decrease in myelin essential protein (MBP) and O4 (a pro-oligodendrocyte marker), as well as a decrease in both the area covered by myelin and reduced thickness of the sheath in the corpus callosum of female rats [19]. This study, however, correlates neuroinflammation with loss of myelination and subsequent cognitive impairment [19]. In addition, oligodendrocyte survival results were dose-dependent after treating cells in culture with cisplatin ([27] reviewed in [77]). The same research group reported similar results in vivo and in vitro models in which decreased oligodendrocyte survival was evidenced by treatment with fluorouracil [39].
These results indicate that chemotherapeutics can also negatively affect the myelination process. This damage appears to be persistent despite the cessation of chemotherapeutics. It would partially explain why some patients report chemobrain symptoms persist years after completing cycles of chemotherapy.
3.1.2. Chemotherapeutics induce indirect mechanisms of neurotoxicity
3.1.2.1. Activation of glial cells
As mentioned before, glial cells are important for CNS functioning. Particularly, astrocytes have been reported as the physical and metabolic support of neurons [53]. However, it has been described that astrocytes are involved in many other processes. In particular, astrocytes have been shown to play a crucial role in cognitive processing by contributing to synaptic transmission and structural plasticity, where bidirectional communication between neurons and astrocytes is established [72]. Accordingly, it has also been reported that astrocytes and microglia play an essential role in the formation and pruning of synapses. Also, it has been reported that the central role of microglia is to phagocytize apoptotic neuronal bodies and some synapses [81]. In recent years, it has been reported that microglia modulates the formation of new neurons in the hippocampus, regulates dendritic and axonal growth, and stimulates the formation, modulation, and relocation of synapses, processes necessary for memory formation [71].
Glial activation is a feature of the immunocompetent cells, such as microglia and astrocytes, in response to injury or toxic agents. Effects of microglial activation after the intraperitoneal administration of cyclophosphamide have been observed in the hippocampus of male rats that show an increase in ED1-positive cells (a marker of microglial activation) in response to treatment; also, this effect seems to be related to impaired performance on novel place recognition (NORT) and contextual fear conditioning tests [26]. In concurrence, in a male murine model, it has been shown that intermittent intraperitoneal application of docetaxel generates an increase in GFAP-positive astrocytes, indicating astrocyte activation. This activation was seen 48 h after docetaxel administration and extended, at lower levels, up to 9 days after application. Furthermore, it was noted that docetaxel-treated animals performed worse in the novel object recognition test [30]. Likewise, in a juvenile murine model, it has been shown that peritoneal application of methotrexate induces microglial activation, which in turn affects oligodendrocyte maturation dynamics and increases astrocyte reactivity. The authors also reported that the reduction of microglia restored tri-glial stability and cognitive function, assessed by the novel object recognition task [34], [35]. Other authors have also shown that administration of DOX-induced astrocytic activation in the frontal cortex, striatum, hippocampus, hypothalamus, and cerebellum. Finally, the authors suggested that these results partially explained the cognitive impairment evidenced by a more unsatisfactory outcome in new object recognition tests [20].
These results reflect the enormous impact of chemotherapeutics on glial populations. Notably, the activation of astrocytes and microglia was evidenced. This is relevant since these processes cause tissue damage due to the release of proinflammatory cytokines that, in turn, generate neuroinflammation and, ultimately, neurodegeneration [65].
3.1.2.2. Neuroinflammation by cytokine dysregulation
Cytokines play a fundamental role in the development and physiology of the CNS. However, the CNS is especially vulnerable to dysregulated cytokine and some changes in the microenvironment due to diseases or external factors. Chemotherapy frequently leads to chronic inflammation that can induce damage to tissue and cell infiltration in the CNS. The mentioned phenomena intertwine, generating a vicious cycle of inflammatory and tissue damage, eventually leading to neurodegeneration [15].
It has been documented that cyclophosphamide in a murine model generates alterations among cytokines. Within the proinflammatory cytokines, interleukin-1β (IL-1β), tumor necrosis factor a (TNFα), and interleukin-6 (IL-6) were found to be significantly elevated in the hippocampus and cortex. In contrast, the anti-inflammatory cytokine IL-10 was significantly decreased. In sum, this imbalance induces a neuroinflammatory environment contributing to cognitive impairment, evidenced by increased immobility time in the forced swim test (FST) and decreased performance in the latency retention transfer test observed in treated animals. These tests evaluate the extent of behaviors associated with depression and memory acquisition and retention [44].
In agreement, it has been reported that the application of cisplatin generates IL-1β, and TNFα, elevation in the hippocampus of a murine model. Along with these results, it was also described that treated animals performed worse in the NORT and motor coordination tests [45]. Similarly, other authors reported in another murine model that the application of cisplatin caused a significant increase in the level of TNFα and IL-6 in the cerebral cortex of the treated group [12]. Likewise, it has been reported that intraperitoneal application of fluorouracil in an aged murine model increased the levels of IL-1β, IL-2, IL-3, IL-4, IL-5, and IL-17 in the hippocampus after one month of treatment, which was related to detrimental effects on mature hippocampal neurons [37].
Furthermore, other researchers have described that chronic intraperitoneal application of paclitaxel in a male murine model elevates levels of the proinflammatory cytokines TNFα and IL-1β in the hippocampus, and these results were related to the subsequent decrease in spatial memory of treated animals by increasing escape latency times in the Morris Water Maze (MWM) test [56]. Notably, treatment with the bleomycin, etoposide, and cisplatin (BEP) regimen in patients with testicular cancer (TC) also significantly increased TNFα levels compared to the group with TC but did not receive BEP treatment. In this study, elevated TNFα levels were associated with poorer cognitive performance [8].
Moreover, the administration of DOX generates a dramatic increase in the levels of the proinflammatory cytokines TNFα and IL-17, while the anti-inflammatory cytokine IL-10 declines in the hippocampus of treated animals. Treated animals also showed reduced short-term memory as measured by the spontaneous alternation test (SAP) [82]. Consistent with this, in another murine model in which DOX was applied intraperitoneally, an overexpression of TNFα levels was evidenced in the brain [78]. Similarly, in a female OVX murine model, it was reported that the application of the regimen doxorubicin and cyclophosphamide (AC) produced in the hippocampus a significant elevation of the cytokines IL-2, IL-3, IL-4, IL-6, IL-10, IL-1ra, IL-17, and TNFα [13].
In addition, it has been reported that using the CMF regimen in an aged female murine model generated increased levels of the proinflammatory cytokines TNFα and IL-1β but reduced levels of the anti-inflammatory cytokine IL-10 in the corpus callosum. Intriguingly, after one month of cessation of treatment with the CMF regimen, the treated animals exhibited worse performance in memory and discrimination tests [19]. Similar results were obtained with the DAC regimen in which levels of the proinflammatory cytokines TNFα and IL-6 were significantly increased, but levels of the anti-inflammatory cytokine IL-4 and IL-10 were decreased in the whole brain, especially in the prefrontal cortex and hippocampus of a female murine model [74].
3.1.2.3. Damage to the blood-brain barrier
Chemotherapeutics promotes an imbalance between proinflammatory and anti-inflammatory cytokines. Pro-inflammatory cytokines, which are overproduced in cancer or in response to chemotherapy, can reduce the integrity of the blood–brain barrier (BBB) and can lead to infiltration of neutrophiles and leukocytes. Remarkable, it was documented that regardless of the chemotherapeutic used, the cytokine frequently found to be elevated as TNFα. This finding is important since it has been described in the literature that the expression of this cytokine is related to increased oxidative stress and mitochondrial dysfunction, which decreased in a TNFα null model [70]. Additionally, it has also been described that the elevation of reactive oxygen species (ROS) and, consequently, of proinflammatory cytokines generate physical damage to the BBB, which subsequently contributes to the entry of small concentrations of chemotherapeutics that generally cannot pass the BBB, as is the case of DOX. In addition, pro-inflammatory molecules such as TNF-α can cross BBB and stimulate microglial cells to produce further inflammatory cytokines, which promote brain damage [70]. On the other hands, a few chemotherapeutic agents can cross the BBB. For example, Methotrexate crosses the blood-brain barrier at high doses; standard doses are generally not associated with neurotoxicity. Nitrosoureas (e.g., carmustine, lomustine) can cross the blood-brain barrier and have been used to treat primary brain tumors (e.g., gliomas) and melanoma, and lymphomas have metastasized to the brain. Cytosine arabinoside is an antimetabolite that crosses the blood-brain barrier. Ifosfamide, an alkylating prodrug, and some of its metabolites, bioactivated by the liver, can penetrate the blood-brain barrier. Unlike cyclophosphamide, ifosfamide is associated with CNS toxicity in 10–30 % of treated patients. Some reported CNS effects include confusion, drowsiness, hallucinations, seizures, and extrapyramidal symptoms [84].
Finally, the described mechanisms show the different effects of chemotherapeutic agents that directly or indirectly affect the communication or function of essential brain regions involved in cognition, such as the prefrontal cortex, hippocampus and striatum [36]. For this reason, further research should continue to explore the short- and long-term effects of chemotherapeutics, as this will also help in the development of therapeutic strategies.
3.2. Future directions
Although different investigations have linked the link between the adverse effects of chemotherapy and cognitive dysfunction, the causes and mechanisms of neurotoxicity are still not well understood. This review examined relevant articles published on this topic to gather knowledge about the mechanisms associated with chemotherapy-induced cognitive impairment. For example, the mechanisms of action of several chemotherapeutic agents have been reported to be related to increased oxidative stress and mitochondrial dysfunction, which causes neuronal damage and therefore affects neurogenesis, neuroplasticity, and cognitive function. In addition, evidence shows that oxidative stress can disrupt the integrity of the blood-brain barrier, allowing neurotoxic substances to enter the brain parenchyma. Therefore, the systemic administration of antioxidant molecules or protectors of mitochondrial activity may be promising in preventing cognitive deterioration. Likewise, plant-derived natural products could potentially prevent or reduce chemotherapy-initiated neurotoxicity. Then, there is a need to explore a possible therapeutic approach to minimize the neurotoxicity induced by systemic chemotherapeutic agents.
On the other hand, CRCI can be caused by an increase in the levels of proinflammatory cytokines, which leads to an increase in inflammation, with effects in the reduction of metabolism in critical regions for learning and memory processes, such as the hippocampus, which can lead to memory impairment. Additionally, it has been reported that the induced inflammatory environment decreases the integrity of the BBB and makes it permeable to neurotoxins. Alternatively, peripheral leukocytes and neutrophils can induce microglia and macroglia activation with increased proinflammatory cytokine production. In this scenario, naturally occurring molecules with anti-inflammatory activity together with combination chemotherapy could be a novel therapeutic approach to prevent the progression of neurotoxicity in patients treated for cancer.
On the other hand, the mechanisms that imply the reduction of neurogenesis, the deregulation of plasticity, and, therefore, neuronal dysfunction still need to be clarified and investigated in future studies since they could shed light on pharmacological targets that allow the development of therapeutic strategies preventative against chemo brain.
Since most of the original articles presented here report results obtained in healthy mouse models, it is essential to validate these results in cancer models, rarely contemplated, to dissect chemotherapy-induced cognitive impairment from the effects induced by the disease itself. In addition, during the review, it became apparent that some murine models used only males or only females or only ovariectomized females. In these cases, valuable information about the effect of chemotherapy in the groups not under consideration could be lost. Therefore, it would be enlightening if more controls could be considered within the experimental design to have a global vision of the results obtained.
4. Conclusion
Cancer-Related Cognitive Impairment is a complex phenomenon mediated by altered neurobiological mechanisms that lead to neurotoxicity, decreasing functionality directly or indirectly. The main neurotoxic effects rely on increased oxidative stress and mitochondrial dysfunction that finally can lead to the loss of neuronal plasticity, inducing decreased dendritic arborization, loss of spines, demyelination, and alterations related to neurotransmission that may cause neurodegeneration. Other mechanisms of damage indirectly caused by the effect of glial cells (macroglia or microglia), such as neuroinflammation and damage to the BBB, are highlighted in the pathophysiology.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank Ministerio de Ciencia, tecnología e Innovación, al Ministerio de Educación Nacional, al Ministerio de Industria, Comercio y Turismo e ICETEX, 2ª Convocatoria Ecosistema científico - Colombia Científica 792–2017, Programa “Generación de alternativas terapéuticas en cáncer a partir de plantas a través de procesos de investigación y desarrollo traslacional, articulados en sistemas de valor sostenibles ambiental y económicamente” (Contrato No. FP44842–221-2018), y Vicerrectoría de investigación, Pontificia Universidad Javeriana, Grant IDs 008111, 006962 y 009725.
Handling Editor: Prof. L.H. Lash
Data availability
Data will be made available on request.
References
- 1.Aggarwal S., Yurlova L., Simons M. Central nervous system myelin: structure, synthesis and assembly. Trends Cell Biol. 2011;21(10):585–593. doi: 10.1016/j.tcb.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Alexander T.C., Simecka C.M., Kiffer F., Groves T., Anderson J., Carr H., Wang J., Carter G., Allen A.R. Changes in cognition and dendritic complexity following intrathecal methotrexate and cytarabine treatment in a juvenile murine model. Behav. Brain Res. 2018;346:21–28. doi: 10.1016/j.bbr.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alhowail A.H., Bloemer J., Majrashi M., Pinky P.D., Bhattacharya S., Yongli Z., Bhattacharya D., Eggert M., Woodie L., Buabeid M.A., Johnson N., Broadwater A., Smith B., Dhanasekaran M., Arnold R.D., Suppiramaniam V. Doxorubicin-induced neurotoxicity is associated with acute alterations in synaptic plasticity, apoptosis, and lipid peroxidation. Toxicol. Mech. Methods. 2019;29(6):457–466. doi: 10.1080/15376516.2019.1600086. [DOI] [PubMed] [Google Scholar]
- 4.Ali M.A., Menze E.T., Tadros M.G., Tolba M.F. Caffeic acid phenethyl ester counteracts doxorubicin-induced chemobrain in Sprague-Dawley rats: emphasis on the modulation of oxidative stress and neuroinflammation. Neuropharmacology. 2020;181 doi: 10.1016/j.neuropharm.2020.108334. [DOI] [PubMed] [Google Scholar]
- 5.Allen B.D., Apodaca L.A., Syage A.R., Markarian M., Baddour A.A.D., Minasyan H., Alikhani L., Lu C., West B.L., Giedzinski E., Baulch J.E., Acharya M.M. Attenuation of neuroinflammation reverses Adriamycin-induced cognitive impairments. Acta Neuropathol. Commun. 2019;7(1):186. doi: 10.1186/s40478-019-0838-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Almeida D., Pinho R., Correia V., Soares J., Bastos M., Carvalho F., Capela J., Costa V. Mitoxantrone is more toxic than doxorubicin in SH-SY5Y human cells: a ‘chemobrain’ in vitro study. Pharmaceuticals. 2018;11(2):41. doi: 10.3390/ph11020041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Cancer Society, 2020. Cancer facts & figures 2020 (p. 76). Atlanta: American Cancer Society. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2020/cancer-facts-and-figures-2020.pdf.
- 8.Amidi A., Agerbæk M., Wu L.M., Pedersen A.D., Mehlsen M., Clausen C.R., Demontis D., Børglum A.D., Harbøll A., Zachariae R. Changes in cognitive functions and cerebral grey matter and their associations with inflammatory markers, endocrine markers, and APOE genotypes in testicular cancer patients undergoing treatment. Brain Imaging Behav. 2017;11(3):769–783. doi: 10.1007/s11682-016-9552-3. [DOI] [PubMed] [Google Scholar]
- 9.Anderson J.E., Trujillo M., McElroy T., Groves T., Alexander T., Kiffer F., Allen A.R. Early effects of cyclophosphamide, methotrexate, and 5-fluorouracil on neuronal morphology and hippocampal-dependent behavior in a murine model. Toxicol. Sci. 2019 doi: 10.1093/toxsci/kfz213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andres A.L., Gong X., Di K., Bota D.A. Low-doses of cisplatin injure hippocampal synapses: a mechanism for ‘chemo’ brain? Exp. Neurol. 2014;255:137–144. doi: 10.1016/j.expneurol.2014.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antkiewicz-Michaluk L., Krzemieniecki K., Romanska I., Michaluk J., Krygowska-Wajs A. Acute treatment with doxorubicin induced neurochemical impairment of the function of dopamine system in rat brain structures. Pharmacol. Rep. 2016;68(3):627–630. doi: 10.1016/j.pharep.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Arafa M.H., Atteia H.H. Protective role of epigallocatechin gallate in a rat model of cisplatin-induced cerebral inflammation and oxidative damage: impact of modulating NF-κB and Nrf2. Neurotox. Res. 2020;37(2):380–396. doi: 10.1007/s12640-019-00095-x. [DOI] [PubMed] [Google Scholar]
- 13.Bagnall-Moreau C., Chaudhry S., Salas-Ramirez K., Ahles T., Hubbard K. Chemotherapy-induced cognitive impairment is associated with increased inflammation and oxidative damage in the hippocampus. Mol. Neurobiol. 2019;56(10):7159–7172. doi: 10.1007/s12035-019-1589-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banach M., Juranek J.K. Chemobrain — a troubling side effect of chemotherapy. J. Depress. Anxiety. 2017;06(03) doi: 10.4172/2167-1044.S11-002. [DOI] [Google Scholar]
- 15.Becher B., Spath S., Goverman J. Cytokine networks in neuroinflammation. Nat. Rev. Immunol. 2017;17(1):49–59. doi: 10.1038/nri.2016.123. [DOI] [PubMed] [Google Scholar]
- 16.Bechler M.E., Swire M., ffrench-Constant C. Intrinsic and adaptive myelination – a sequential mechanism for smart wiring in the brain: Intrinsic and Adaptive Myelination Mechanisms. Dev. Neurobiol. 2018;78(2):68–79. doi: 10.1002/dneu.22518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhattacharya B., Mukherjee S. Cancer therapy using antibiotics. J. Cancer Ther. 2015;06(10):849–858. doi: 10.4236/jct.2015.610093. [DOI] [Google Scholar]
- 18.Briones T.L., Woods J. Chemotherapy-induced cognitive impairment is associated with decreases in cell proliferation and histone modifications. BMC Neurosci. 2011;12(1):124. doi: 10.1186/1471-2202-12-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Briones T.L., Woods J. Dysregulation in myelination mediated by persistent neuroinflammation: possible mechanisms in chemotherapy-related cognitive impairment. Brain, Behav., Immun. 2014;35:23–32. doi: 10.1016/j.bbi.2013.07.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cardoso C.V., de Barros M.P., Bachi A.L.L., Bernardi M.M., Kirsten T.B., de Fátima Monteiro Martins M., Rocha P.R.D., da Silva Rodrigues P., Bondan E.F. Chemobrain in rats: behavioral, morphological, oxidative and inflammatory effects of doxorubicin administration. Behav. Brain Res. 2020;378 doi: 10.1016/j.bbr.2019.112233. [DOI] [PubMed] [Google Scholar]
- 21.Castel H., Denouel A., Lange M., Tonon M.-C., Dubois M., Joly F. Biomarkers associated with cognitive impairment in treated cancer patients: potential predisposition and risk factors. Front. Pharmacol. 2017;8 doi: 10.3389/fphar.2017.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheung-Ong K., Giaever G., Nislow C. DNA-damaging agents in cancer chemotherapy: serendipity and chemical biology. Chem. Biol. 2013;20(5):648–659. doi: 10.1016/j.chembiol.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 23.Chidambaram S.B., Rathipriya A.G., Bolla S.R., Bhat A., Ray B., Mahalakshmi A.M., Manivasagam T., Thenmozhi A.J., Essa M.M., Guillemin G.J., Chandra R., Sakharkar M.K. Dendritic spines: revisiting the physiological role. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2019;92:161–193. doi: 10.1016/j.pnpbp.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 24.Chiorazzi A., Semperboni S., Marmiroli P. Current view in platinum drug mechanisms of peripheral neurotoxicity. Toxics. 2015;3(3):304–321. doi: 10.3390/toxics3030304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiu G.S., Maj M.A., Rizvi S., Dantzer R., Vichaya E.G., Laumet G., Kavelaars A., Heijnen C.J. Pifithrin-μ prevents cisplatin-induced chemobrain by preserving neuronal mitochondrial function. Cancer Res. 2017;77(3):742–752. doi: 10.1158/0008-5472.CAN-16-1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christie L.-A., Acharya M.M., Parihar V.K., Nguyen A., Martirosian V., Limoli C.L. Impaired cognitive function and hippocampal neurogenesis following cancer chemotherapy. Clin. Cancer Res. 2012;18(7):1954–1965. doi: 10.1158/1078-0432.CCR-11-2000. [DOI] [PubMed] [Google Scholar]
- 27.Dietrich J., Han R., Yang Y., Mayer-Pröschel M., Noble M. CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo. J. Biol. 2006;5(7):22. doi: 10.1186/jbiol50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.English K., Shepherd A., Uzor N.-E., Trinh R., Kavelaars A., Heijnen C.J. Astrocytes rescue neuronal health after cisplatin treatment through mitochondrial transfer. Acta Neuropathol. Commun. 2020;8(1):36. doi: 10.1186/s40478-020-00897-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faber D.S., Pereda A.E. Two forms of electrical transmission between neurons. Front. Mol. Neurosci. 2018;11:427. doi: 10.3389/fnmol.2018.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fardell J.E., Zhang J., De Souza R., Vardy J., Johnston I., Allen C., Henderson J., Piquette-Miller M. The impact of sustained and intermittent docetaxel chemotherapy regimens on cognition and neural morphology in healthy mice. Psychopharmacology. 2014;231(5):841–852. doi: 10.1007/s00213-013-3301-8. [DOI] [PubMed] [Google Scholar]
- 31.Faria O., Gonsalvez D.G., Nicholson M., Xiao J. Activity‐dependent central nervous system myelination throughout life. J. Neurochem. 2019;148(4):447–461. doi: 10.1111/jnc.14592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flanigan T.J., Anderson J.E., Elayan I., Allen A.R., Ferguson S.A. Effects of cyclophosphamide and/or doxorubicin in a murine model of postchemotherapy cognitive impairment. Toxicol. Sci. 2018;162(2):462–474. doi: 10.1093/toxsci/kfx267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fouad G.I., Ahmed K.A. Neuroprotective potential of berberine against doxorubicin-induced toxicity in rat's brain. Neurochem. Res. 2021;46(12):3247–3263. doi: 10.1007/s11064-021-03428-5. http://doi.org10.1007/s11064-021-03428-5. [DOI] [PubMed] [Google Scholar]
- 34.Geraghty A.C., Gibson E.M., Ghanem R.A., Greene J.J., Ocampo A., Goldstein A.K., Ni L., Yang T., Marton R.M., Paşca S.P., Greenberg M.E., Longo F.M., Monje M. Loss of adaptive myelination contributes to methotrexate chemotherapy-related cognitive impairment. Neuron. 2019;103(2):250–265. doi: 10.1016/j.neuron.2019.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gibson E.M., Nagaraja S., Ocampo A., Tam L.T., Wood L.S., Pallegar P.N., Greene J.J., Geraghty A.C., Goldstein A.K., Ni L., Woo P.J., Barres B.A., Liddelow S., Vogel H., Monje M. Methotrexate chemotherapy induces persistent tri-glial dysregulation that underlies chemotherapy-related cognitive impairment. Cell. 2019;176(1–2):43–55. doi: 10.1016/j.cell.2018.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gonzalez-Burgos I., Feriavelasco A. Serotonin/dopamine interaction in memory formation. Prog. Brain Res. 2008:603–623. doi: 10.1016/S0079-6123(08)00928-X. [DOI] [PubMed] [Google Scholar]
- 37.Groves T.R., Farris R., Anderson J.E., Alexander T.C., Kiffer F., Carter G., Wang J., Boerma M., Allen A.R. 5-fluorouracil chemotherapy upregulates cytokines and alters hippocampal dendritic complexity in aged mice. Behav. Brain Res. 2017;316:215–224. doi: 10.1016/j.bbr.2016.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Habli Z., Toumieh G., Fatfat M., Rahal O., Gali-Muhtasib H. Emerging cytotoxic alkaloids in the battle against cancer: overview of molecular mechanisms. Molecules. 2017;22(2):250. doi: 10.3390/molecules22020250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han R., Yang Y.M., Dietrich J., Luebke A., Mayer-Pröschel M., Noble M. Systemic 5-fluorouracil treatment causes a syndrome of delayed myelin destruction in the central nervous system. J. Biol. 2008;7(4):12. doi: 10.1186/jbiol69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 41.Handra C. The connection between different neurotransmitters involved in cognitive processes. Farmacia. 2019;67(2):193–201. doi: 10.31925/farmacia.2019.2.1. [DOI] [Google Scholar]
- 42.Huehnchen P., Boehmerle W., Springer A., Freyer D., Endres M. A novel preventive therapy for paclitaxel-induced cognitive deficits: preclinical evidence from C57BL/6 mice. Transl. Psychiatry. 2017;7(8) doi: 10.1038/tp.2017.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ikonomidou C. Chemotherapy and the pediatric brain. Mol. Cell. Pediatr. 2018;5(1):8. doi: 10.1186/s40348-018-0087-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iqubal A., Sharma S., Najmi A.K., Syed M.A., Ali J., Alam M.M., Haque S.E. Nerolidol ameliorates cyclophosphamide-induced oxidative stress, neuroinflammation and cognitive dysfunction: plausible role of Nrf2 and NF- κB. Life Sci. 2019;236 doi: 10.1016/j.lfs.2019.116867. [DOI] [PubMed] [Google Scholar]
- 45.Jangra A., Kwatra M., Singh T., Pant R., Kushwah P., Ahmed S., Dwivedi D., Saroha B., Lahkar M. Edaravone alleviates cisplatin-induced neurobehavioral deficits via modulation of oxidative stress and inflammatory mediators in the rat hippocampus. Eur. J. Pharmacol. 2016;791:51–61. doi: 10.1016/j.ejphar.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 46.Jarmolowicz D.P., Gehringer R., Lemley S.M., Sofis M.J., Kaplan S., Johnson M.A. 5-Fluorouracil impairs attention and dopamine release in rats. Behav. Brain Res. 2019;362:319–322. doi: 10.1016/j.bbr.2019.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ježek J., Cooper K.F., Strich R. The impact of mitochondrial fission-stimulated ROS production on pro-apoptotic chemotherapy. Biology. 2021;10(1):33. doi: 10.3390/biology10010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Joly F., Giffard B., Rigal O., De Ruiter M.B., Small B.J., Dubois M., LeFel J., Schagen S.B., Ahles T.A., Wefel J.S., Vardy J.L., Pancré V., Lange M., Castel H. Impact of cancer and its treatments on cognitive function: advances in research from the paris international cognition and cancer task force symposium and update since 2012. J. Pain. Symptom Manag. 2015;50(6):830–841. doi: 10.1016/j.jpainsymman.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 49.Kaplan S.V., Limbocker R.A., Gehringer R.C., Divis J.L., Osterhaus G.L., Newby M.D., Sofis M.J., Jarmolowicz D.P., Newman B.D., Mathews T.A., Johnson M.A. Impaired brain dopamine and serotonin release and uptake in wistar rats following treatment with carboplatin. ACS Chem. Neurosci. 2016;7(6):689–699. doi: 10.1021/acschemneuro.5b00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kärkkäinen V., Pomeshchik Y., Savchenko E., Dhungana H., Kurronen A., Lehtonen S., Naumenko N., Tavi P., Levonen A.-L., Yamamoto M., Malm T., Magga J., Kanninen K.M., Koistinaho J. Nrf2 regulates neurogenesis and protects neural progenitor cells against Aβ toxicity: regulation and protection of NPCs by Nrf2. Stem Cells. 2014;32(7):1904–1916. doi: 10.1002/stem.1666. [DOI] [PubMed] [Google Scholar]
- 51.Kasai H., Fukuda M., Watanabe S., Hayashi-Takagi A., Noguchi J. Structural dynamics of dendritic spines in memory and cognition. Trends Neurosci. 2010;33(3):121–129. doi: 10.1016/j.tins.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 52.Kempermann G., Song H., Gage F.H. Neurogenesis in the adult hippocampus. Cold Spring Harb. Perspect. Biol. 2015;7(9):a018812. doi: 10.1101/cshperspect.a018812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kıray H., Lindsay S.L., Hosseinzadeh S., Barnett S.C. The multifaceted role of astrocytes in regulating myelination. Exp. Neurol. 2016;283:541–549. doi: 10.1016/j.expneurol.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kovalchuk A., Ilnytskyy Y., Woycicki R., Rodriguez-Juarez R., Metz G.A.S., Kovalchuk O. Adverse effects of paternal chemotherapy exposure on the progeny brain: intergenerational chemobrain. Oncotarget. 2018;9(11):10069–10082. doi: 10.18632/oncotarget.24311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Levin E.D., editor. Birkhäuser Verlag; 2006. Neurotransmitter Interactions and Cognitive Function. [Google Scholar]
- 56.Li Z., Zhao S., Zhang H.-L., Liu P., Liu F.-F., Guo Y.-X., Wang X.-L. Proinflammatory factors mediate paclitaxel-induced impairment of learning and memory. Mediat. Inflamm. 2018;2018:1–9. doi: 10.1155/2018/3941840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liao D., Xiang D., Dang R., Xu P., Wang J., Han W., Fu Y., Yao D., Cao L., Jiang P. Neuroprotective effects of dl-3-n-butylphthalide against doxorubicin-induced neuroinflammation, oxidative stress, endoplasmic reticulum stress, and behavioral changes. Oxid. Med. Cell. Longev. 2018;2018:1–13. doi: 10.1155/2018/9125601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P.A., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009;62(10):e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 59.Magge R.S., DeAngelis L.M. The double-edged sword: neurotoxicity of chemotherapy. Blood Rev. 2015;29(2):93–100. doi: 10.1016/j.blre.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meléndez D.M., Nordquist R.E., Vanderschuren L.J.M.J., van der Staay F.-J. Spatial memory deficits after vincristine-induced lesions to the dorsal hippocampus. PLOS One. 2020;15(4) doi: 10.1371/journal.pone.0231941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moore K., Stutzman S., Priddy L., Olson D. Chemobrain: a pilot study exploring the severity and onset of chemotherapy-related cognitive impairment. Clin. J. Oncol. Nurs. 2019;23(4):411–416. doi: 10.1188/19.CJON.411-416. [DOI] [PubMed] [Google Scholar]
- 62.Mustafa S., Walker A., Bennett G., Wigmore P.M. 5-Fluorouracil chemotherapy affects spatial working memory and newborn neurons in the adult rat hippocampus. Eur. J. Neurosci. 2008;28(2):323–330. doi: 10.1111/j.1460-9568.2008.06325.x. [DOI] [PubMed] [Google Scholar]
- 63.Myers J.S., Pierce J., Pazdernik T. Neurotoxicology of chemotherapy in relation to cytokine release, the blood-brain barrier, and cognitive impairment. Oncol. Nurs. Forum. 2008;35(6):916–920. doi: 10.1188/08.ONF.916-920. [DOI] [PubMed] [Google Scholar]
- 64.Nguyen L.D., Ehrlich B.E. Cellular mechanisms and treatments for chemobrain: Insight from aging and neurodegenerative diseases. EMBO Mol. Med. 2020;12(6) doi: 10.15252/emmm.202012075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pekny M., Wilhelmsson U., Pekna M. The dual role of astrocyte activation and reactive gliosis. Neurosci. Lett. 2014;565:30–38. doi: 10.1016/j.neulet.2013.12.071. [DOI] [PubMed] [Google Scholar]
- 66.Pellacani C., Eleftheriou G. Neurotoxicity of antineoplastic drugs: mechanisms, susceptibility, and neuroprotective strategies. Adv. Med. Sci. 2020;65(2):265–285. doi: 10.1016/j.advms.2020.04.001. [DOI] [PubMed] [Google Scholar]
- 67.Piccolini V.M., Esposito A., Dal Bo V., Insolia V., Bottone M.G., De Pascali S.A., Fanizzi F.P., Bernocchi G. Cerebellum neurotransmission during postnatal development: [Pt( O, O ′‐acac)(γ‐acac)(DMS)] vs cisplatin and neurotoxicity. Int. J. Dev. Neurosci. 2015;40(1):24–34. doi: 10.1016/j.ijdevneu.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 68.Ramalingayya G.V., Cheruku S.P., Nayak P.G., Kishore A., Shenoy R., et al. Rutin protects against neuronal damage in vitro and ameliorates doxorubicin-induced memory deficits in vivo in Wistar rats. Drug Des. Dev. Ther. 2017;11:1011–1026. doi: 10.2147/DDDT.S103511. http://doi.org10.2147/DDDT.S103511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ren X., Boriero D., Chaiswing L., Bondada S., St., Clair D.K., Butterfield D.A. Plausible biochemical mechanisms of chemotherapy-induced cognitive impairment (“chemobrain”), a condition that significantly impairs the quality of life of many cancer survivors. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 2019;1865(6):1088–1097. doi: 10.1016/j.bbadis.2019.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ren X., Keeney J.T.R., Miriyala S., Noel T., Powell D.K., Chaiswing L., Bondada S., St., Clair D.K., Butterfield D.A. The triangle of death of neurons: Oxidative damage, mitochondrial dysfunction, and loss of choline-containing biomolecules in brains of mice treated with doxorubicin. Advanced insights into mechanisms of chemotherapy induced cognitive impairment (“chemobrain”) involving TNF-α. Free Radic. Biol. Med. 2019;134:1–8. doi: 10.1016/j.freeradbiomed.2018.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rodríguez-Iglesias N., Sierra A., Valero J. Rewiring of memory circuits: connecting adult newborn neurons with the help of microglia. Front. Cell Dev. Biol. 2019;7:24. doi: 10.3389/fcell.2019.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Santello M., Toni N., Volterra A. Astrocyte function from information processing to cognition and cognitive impairment. Nat. Neurosci. 2019;22(2):154–166. doi: 10.1038/s41593-018-0325-8. [DOI] [PubMed] [Google Scholar]
- 73.Shaker F.H., El-Derany M.O., Wahdan S.A., El-Demerdash E., El-Mesallamy H.O. Berberine ameliorates doxorubicin-induced cognitive impairment (chemobrain) in rats. Life Sci. 2021;269 doi: 10.1016/j.lfs.2021.119078. [DOI] [PubMed] [Google Scholar]
- 74.Shi D.-D., Huang Y.-H., Lai C.S.W., Dong C.M., Ho L.C., Wu E.X., Li Q., Wang X.-M., Chung S.K., Sham P.C., Zhang Z.-J. Chemotherapy-induced cognitive impairment is associated with cytokine dysregulation and disruptions in neuroplasticity. Mol. Neurobiol. 2019;56(3):2234–2243. doi: 10.1007/s12035-018-1224-4. [DOI] [PubMed] [Google Scholar]
- 75.Singh S., Kumar A. Protective effect of edaravone on cyclophosphamide induced oxidative stress and neurotoxicity in rats. Curr. Drug Saf. 2019;14(3):209–216. doi: 10.2174/1574886314666190506100717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Snyder J.S. Recalibrating the relevance of adult neurogenesis. Trends Neurosci. 2019;42(3):164–178. doi: 10.1016/j.tins.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 77.Stankovic J.S.K., Selakovic D., Mihailovic V., Rosic G. Antioxidant supplementation in the treatment of neurotoxicity induced by platinum-based chemotherapeutics – a review. Int. J. Mol. Sci. 2020;21(20):7753. doi: 10.3390/ijms21207753. https://doi.org/10.3390/ijms21207753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tabaczar S., Czepas J., Koceva A., Kilanczyk E., Piasecka-Zelga J., Gwozdzinski K. The effect of the nitroxide pirolin on oxidative stress induced by doxorubicin and taxanes in the rat brain. J. Physiol. Pharmacol. 2017;68:295–308. [PubMed] [Google Scholar]
- 79.Thomas T.C., Beitchman J.A., Pomerleau F., Noel T., Jungsuwadee P., Butterfield D.A., Clair D.K., St., Vore M., Gerhardt G.A. Acute treatment with doxorubicin affects glutamate neurotransmission in the mouse frontal cortex and hippocampus. Brain Res. 2017;1672:10–17. doi: 10.1016/j.brainres.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Torre M., Dey A., Woods J.K., Feany M.B. Elevated oxidative stress and DNA damage in cortical neurons of chemotherapy patients. J. Neuropathol. Exp. Neurol. 2021;80(7):705–712. doi: 10.1093/jnen/nlab074. http://doi.org10.1093/jnen/nlab074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vainchtein I.D., Molofsky A.V. Astrocytes and microglia: in sickness and in health. Trends Neurosci. 2020;43(3):144–154. doi: 10.1016/j.tins.2020.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wahdan S.A., El-Derany M.O., Abdel-Maged A.E., Azab S.S. Abrogating doxorubicin-induced chemobrain by immunomodulators IFN-beta 1a or infliximab: Insights to neuroimmune mechanistic hallmarks. Neurochem. Int. 2020;138 doi: 10.1016/j.neuint.2020.104777. [DOI] [PubMed] [Google Scholar]
- 83.Wang C., Zhao Y., Wang L., Pan S., Liu Y., Li S., Wang D. C-phycocyanin mitigates cognitive impairment in doxorubicin-induced chemobrain: impact on neuroinflammation, oxidative stress, and brain mitochondrial and synaptic alterations. Neurochem. Res. 2021;46(2):149–158. doi: 10.1007/s11064-020-03164-2. [DOI] [PubMed] [Google Scholar]
- 84.Wardill H.R., Mander K.A., Van Sebille Y.Z., Gibson R.J., Logan R.M., Bowen J.M., Sonis S.T. Cytokine-mediated blood brain barrier disruption as a conduit for cancer/chemotherapy-associated neurotoxicity and cognitive dysfunction. Int. J. Cancer. 2016;139(12):2635–2645. doi: 10.1002/ijc.30252. http://doi.org10.1002/ijc.30252. [DOI] [PubMed] [Google Scholar]
- 85.Welbat J.U., Naewla S., Pannangrong W., Sirichoat A., Aranarochana A., Wigmore P. Neuroprotective effects of hesperidin against methotrexate-induced changes in neurogenesis and oxidative stress in the adult rat. Biochem. Pharmacol. 2020;178 doi: 10.1016/j.bcp.2020.114083. [DOI] [PubMed] [Google Scholar]
- 86.Wen J., Chen W., Zhu Y., Zhang P. Clinical features associated with the efficacy of chemotherapy in patients with glioblastoma (GBM): a surveillance, epidemiology, and end results (SEER) analysis. BMC Cancer. 2021;21(1):81. doi: 10.1186/s12885-021-07800-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.World Health Organization . International Agency for Research on Cancer,; 2020. All Cancers. [Google Scholar]
- 88.Zajączkowska R., Kocot-Kępska M., Leppert W., Wrzosek A., Mika J., Wordliczek J. Mechanisms of chemotherapy-induced peripheral neuropathy. Int. J. Mol. Sci. 2019;20(6):1451. doi: 10.3390/ijms20061451. https://doi.org10.3390/ijms20061451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhou W., Kavelaars A., Heijnen C.J. Metformin prevents cisplatin-induced cognitive impairment and brain damage in mice. PLOS One. 2016;11(3) doi: 10.1371/journal.pone.0151890. e0151890. http://doi.org10.1371/journal.pone.0151890. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.