Abstract
Objectives
Differences in indication and technique make a randomised comparison between valve-sparing root replacement (VSRR) and personalised external aortic root support (PEARS) challenging. We performed a propensity score (PS)-matched comparison of PEARS and VSRR for syndromic root aneurysm.
Methods
Patients in the PEARS 200 Database and Aortic Valve Insufficiency and ascending aorta Aneurysm InternATiOnal Registry (undergoing VSRR) with connective tissue disease operated electively for root aneurysm <60 mm with aortic regurgitation (AR) <1/4 were included. Using a PS analysis, 80 patients in each cohort were matched. Survival, freedom from reintervention and from AR ≥2/4 were estimated using a Kaplan-Meier analysis.
Results
Median follow-up was 25 and 55 months for 159 PEARS and 142 VSRR patients. Seven (4.4%) patients undergoing PEARS required an intervention for coronary injury or impingement, resulting in one death (0.6%). After VSRR, there were no early deaths, 10 (7%) reinterventions for bleeding and 1 coronary intervention. Survival for matched cohorts at 5 years was similar (PEARS 98% vs VSRR 99%, p=0.99). There was no difference in freedom from valve or ascending aortic/arch reintervention between matched groups. Freedom from AR ≥2/4 at 5 years in the matched cohorts was 97% for PEARS vs 92% for VSRR (p=0.55). There were no type A dissections.
Conclusions
VSRR and PEARS offer favourable mid-term survival, freedom from reintervention and preservation of valve function. Both treatments deserve their place in the surgical repertoire, depending on a patient’s disease stage. This study is limited by its retrospective nature and different follow-ups in both cohorts.
Keywords: Aortic Aneurysm, Marfan Syndrome, Aortic Valve Insufficiency
WHAT IS ALREADY KNOWN ON THIS TOPIC
While valve-sparing root replacement (VSRR) is the established treatment for syndromic root aneurysm, there remains a cumulative risk of aortic valve reintervention. In personalised external aortic root support (PEARS), the dilated aorta is supported using a bespoke mesh, optimally respecting valve anatomy. To date, no type A dissections have been observed after PEARS.
WHAT THIS STUDY ADDS
VSRR and PEARS both seem to offer favourable mid-term survival, freedom from reintervention and preservation of valve function in syndromic root aneurysm with near-normal valve function. Our study indicates that an earlier intervention in the disease progression via PEARS may be justified with a low probability of developing aortic events if the necessary attention goes to the coronary anatomy.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Long-term data are needed to determine the role of all possible treatments in the surgical repertoire. Improved prediction of aortic complications will further help guide patient selection. A shared decision-making process should involve weighing the risk of watchful waiting against the potential risks of all available surgical treatments.
VSRR and PEARS both seem to offer favourable mid-term survival, freedom from reintervention and preservation of valve function in syndromic root aneurysm with near-normal valve function. Our study indicates that an earlier intervention in the disease progression via PEARS may be justified with a low probability of developing aortic events if the necessary attention goes to the coronary anatomy.
Introduction
Valve-sparing root replacement (VSRR) is an established surgical treatment for aortic root aneurysm.1 Depending on patient selection and surgeon experience, excellent freedom from reintervention with a low incidence of valve-related events can be achieved in Marfan syndrome (MFS) and other connective tissue diseases (CTDs).2 There remains, however, a cumulative risk of reintervention on the aortic valve and distal aorta.3 4 Personalised external aortic root support (PEARS) is a total tissue-preserving alternative to VSRR which involves the use of a bespoke mesh, the ExoVasc implant (figure 1), to stabilise the aorta from the ventriculoaortic junction to the origin of the brachiocephalic trunk.5 6 This emerging procedure has been applied primarily in patients with syndromic root aneurysm between 40 and 50 mm and at most mild (grade 1/4) aortic regurgitation (AR) as previously reported in Heart in 2014 and 2021.6 7 Because the dilated aorta is left in place, patients undergoing PEARS are typically operated in an earlier disease stage than if they would undergo VSRR. No dissections have been observed in the supported segment of aorta yet continued follow-up is needed.7 Differences in indication, timing and surgical technique make it difficult to define a group of patients where there is equipoise for a randomised comparison between PEARS and VSRR.8 PEARS is typically a pre-emptive procedure performed in the preclinical phase, while VSRR is an established prophylactic operation performed when a diameter threshold is met.1 We aimed to compare demographics and outcomes of patients undergoing PEARS and VSRR for syndromic root aneurysm with at most mild AR and to perform a propensity score (PS)-matched analysis to discover the magnitude of difference for available outcome measures.
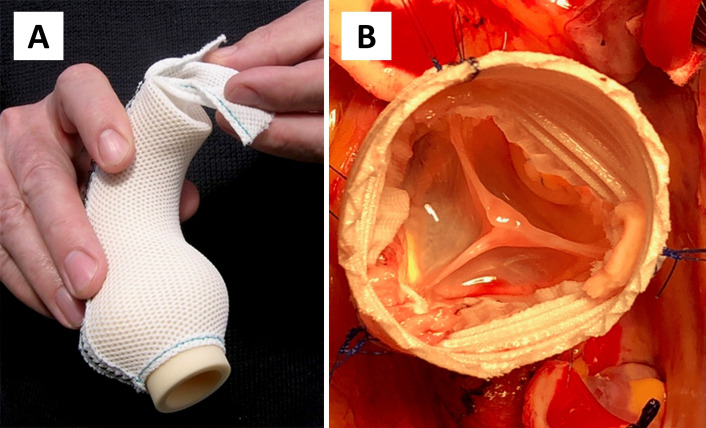
Figure 1.
(A) ExoVasc implant used to stabilise the ascending aorta during personalised external aortic root support surgery. (B) Intraoperative photograph of valve-sparing root replacement. The corrugations of the relatively rigid structure of the low-porosity vascular graft can be seen. Illustration provided by Exstent.
Methods
Study design
We present a multicentre study using two existing databases: the ‘PEARS 200’ and ‘AVIATOR’ Database (Aortic Valve Insufficiency and ascending aorta Aneurysm InternATiOnal Registry), both containing prospectively and retrospectively collected data.7 9 The AVIATOR Project was initiated by the Heart Valve Society as an international registry collecting data on patients undergoing surgery on the proximal aorta, with patients consenting.9 A proposal to the AVIATOR scientific committee request permission for a subgroup analysis (RP#13) was approved. Perioperative outcomes on all patients undergoing PEARS are collected prospectively by Exstent. In the context of an earlier study, we retrospectively collected clinical follow-up data for the first 200 consecutive PEARS operations for root aneurysm worldwide.7
Data collection and study endpoints
From both databases, patients with CTD (MFS, Loeys-Dietz syndrome and ACTA2 mutations) undergoing elective surgery for aortic root aneurysm <60 mm diameter with at most mild AR (grade 0/4 or 1/4) were extracted. Patients with ascending aortic dissection or endocarditis were excluded. The primary endpoint was mid-term survival. Secondary endpoints were in-hospital mortality and the occurrence of postoperative complications (reintervention for bleeding, myocardial infarction, need for coronary revascularisation, stroke, perioperative dissection). Secondary endpoints during follow-up were the occurrence of type A and type B dissections, freedom from valve reintervention, freedom from valve or aortic intervention (ascending aorta and arch) and freedom from AR grade ≥2/4.
Data analysis
Continuous variables were tested for normality with the Shapiro-Wilk test, shown as median (IQR) and compared using Mann-Whitney U test. Categorical variables were shown as n (%) and compared via Χ2 or Fisher’s exact test. Matched data were compared using the Wilcoxon signed-rank test or the McNemar’s (-Bowker) test for related samples. To adjust for potential confounders when comparing PEARS and VSRR, 1:1 PS matching was performed. We chose a PS analysis as it would yield two real populations after matching, rather than using a matching technique which would provide outcomes on a pseudo-population. Furthermore, we believed this strategy would help us better understand the overlap and discrepancies between the patients undergoing PEARS and VSRR. Gender, age, height, weight, a history of cardiac surgery, EuroSCORE II, left ventricular ejection fraction, maximal aortic root diameter, preoperative AR grade and scheduled concomitant procedure were used to determine individual PS, thereby including variables which we considered to be related to the underlying CTD severity. Underlying aneurysm aetiology was not used to match to avoid excluding patients with a less common CTD. There were no missing data among the covariates used in the PS model. Patients were matched using the ‘MatchIt’ package in R studio, performing a logistic regression to calculate the PS and matched with the nearest neighbour method, without replacement and a calliper width of 0.1 of the pooled SD of the logit PS. A calliper width of 0.1 was chosen as it resulted in optimal matching with standardised mean differences below 0.1 for all covariates as well as for the overall PS. Statistical significance was set at p<0.05. Survival, freedom from reintervention and freedom from AR ≥2/4 during follow-up were estimated using a Kaplan-Meier analysis with comparison between groups via a log-rank (Mantel-Cox) test. For patients undergoing PEARS, there was one echocardiography registration during follow-up and for VSRR patients there were between 1 and 26. Patients were censored after their last echocardiography and considered to have no occurrence of AR if there was no registration in the interval between registrations. Data analysis was performed using Microsoft Office Excel V.2016 (Microsoft), SPSS Statistics V26.0 (IBM) and RStudio (RStudio, PBC) and Prism (GraphPad Software).
Patient and public involvement
No patients were involved in the design, conduct or reporting of this study.
Results
Baseline demographics
The 159 included PEARS patients were operated at 20 centres between 2004 and 2019, while the 142 patients undergoing VSRR from the AVIATOR underwent surgery at 13 centres between 1996 and 2021. Eighty patients in each cohort were matched using a PS analysis. Before matching, patients undergoing VSRR were significantly older, more likely to have mild AR or a history of cardiac surgery, had a higher EuroSCORE II and larger aortic root. After matching, covariates were balanced (table 1).
Table 1.
Demographics for the total population and matched population
| Demographics | Unmatched patients | Propensity score-matched patients | ||||||
| PEARS (n=159) | VSRR (n=142) | SMD | P value | PEARS (n=80) | VSRR (n=80) | SMD | P value | |
| Age (years) | 31 (22–40) | 33 (26–41) | 0.24 | 0.06 | 31.7 (21.5–42.5) | 32 (26.25–38) | 0.08 | 0.63 |
| Male | 101 (63.5%) | 98 (69%) | 0.12 | 0.32 | 57 (71.3%) | 57 (71.3%) | 0.00 | 1.00 |
| Height (cm) | 186 (180–193) | 186 (180–194) | 0.05 | 0.91 | 188 (182–194) | 189 (182–194) | 0.09 | 0.97 |
| Aetiology | 0.21 | <0.01 | ||||||
| Marfan | 142 (89.3%) | 117 (82.4%) | 77 (96.3%) | 64 (80.0%) | ||||
| Loeys-Dietz | 15 (9.4%) | 23 (16.2%) | 2 (2.5%) | 15 (18.8%) | ||||
| ACTA2 mutation | 2 (1.3%) | 2 (1.4%) | 1 (1.3%) | 1 (1.3%) | ||||
| EuroSCORE II (%) | 1.0 (1.0–1.2) | 1.6 (1.0–2.1) | 0.43 | <0.001 | 1.0 (1.0–1.2) | 1.2 (1.0–1.6) | 0.09 | 0.35 |
| Previous cardiac surgery | 3 (1.9%) | 14 (9.9%) | 0.27 | <0.01 | 2 (2.5%) | 3 (3.8%) | 0.04 | 1.00 |
| Root diameter (mm) | 46 (43–48) | 49 (46–50) | 0.84 | <0.001 | 48 (46–50) | 48 (46–49) | −0.01 | 0.12 |
| Preoperative AR grade | 0.41 | <0.001 | −0.03 | 1.00 | ||||
| 0/4 | 123 (77.4%) | 81 (57%) | 55 (68.8%) | 56 (70.0%) | ||||
| 1/4 | 36 (22.6%) | 61 (43%) | 25 (31.3%) | 24 (30.0%) | ||||
| LVEF (%) | −0.06 | 0.65 | 0.00 | 1.00 | ||||
| Good >50% | 148 (93.1%) | 134 (94.4%) | 76 (95.0%) | 76 (95.0%) | ||||
| Moderate (31%–50%) | 11 (6.9%) | 8 (5.6%) | 4 (5.0%) | 4 (5.0%) | ||||
| Concomitant procedure planned | 21 (13.2%) | 26 (18.3%) | 0.13 | 0.22 | 12 (15.0%) | 13 (16.3%) | 0.03 | 1.00 |
Underlying aetiology was not used to match. Continuous variables were compared using Mann-Whitney U test, categorical variables via Χ2 or Fisher’s exact test. Matched data were compared using the Wilcoxon signed-rank test or the McNemar’s test for related samples.
SMD was used to evaluate balance between groups.
AR, aortic regurgitation; LVEF, left ventricular ejection fraction; PEARS, personalised external aortic root support; SMD, standardised mean difference; VSRR, valve-sparing root replacement.
Operative variables and in-hospital outcomes
In both groups, concomitant procedures predominantly involved the mitral valve (12.3%, 20 of 159 for PEARS and 11.3%, 16 of 142 for VSRR). Cardiopulmonary bypass was used in 18.3% (24 of 131) of uncomplicated isolated aortic PEARS cases. For patients undergoing VSRR, 57% were operated via the reimplantation/David technique and 37.4% underwent remodelling with aortic annuloplasty. Average aortic cross-clamp time was 138±33 min with nine patients requiring an additional clamping session. Nearly two-thirds (65.5%) of patients undergoing VSRR did not require cusp repair, while one cusp was repaired in 21.1%, most commonly via central free-margin plication. An overview of operative variables is shown in table 2.
Table 2.
Operative variables
| PEARS (n=159) | |
| PEARS completed | 156 (98.1) |
| Isolated aortic PEARS | 136 (85.5) |
| PEARS+mitral valve repair | 18 (11.3) |
| PEARS+elective CABG | 1 (0.6) |
| PEARS+mitral valve replacement | 1 (0.6) |
| Converted to VSRR (1 with mitral valve repair) | 2 (1.3) |
| Procedure aborted | 1 (0.6) |
| Implant size (n=156) | |
| Scaled to 95% luminal diameter | 77 (49.9) |
| Scaled to 100% luminal diameter | 79 (50.6) |
| Completed PEARS procedures (n=156) | |
| Operative duration (min) | 169 (145–204) |
| Isolated aortic PEARS (n=136) | |
| Operative duration (min) | 164 (144–200) |
| CPB used | 29 (21.3) |
| CPB time (min) | 60 (41–77) |
| VSRR (n=142) | |
| Operative technique | |
| Reimplantation (David) | 81 (57) |
| Remodelling+external annuloplasty | 53 (37.4) |
| Remodelling (Yacoub) | 8 (5.6) |
| Aortic leaflet procedures | |
| Tricuspid aortic valve | 139 (97.9) |
| No repair | 92 (64.8) |
| 1 cusp repaired | 28 (19.7) |
| 2 cusps repaired | 11 (7.8) |
| 3 cusps repaired | 8 (5.6) |
| Bicuspid aortic valve | 3 (2.1) |
| No repair | 1 (0.7) |
| 1 cusp repaired | 2 (1.4) |
| Undergoing concomitant procedure | 26 (18.3) |
| Total concomitant procedures | 29 (20.4) |
| Mitral valve repair | 16 (11.3) |
| PFO closure | 5 (3.5) |
| CABG | 4 (2.8) |
| Ablation | 2 (1.4) |
| Extra-anatomical coeliac trunk bypass | 1 (0.7) |
| Tricuspid valve annuloplasty | 1 (0.7) |
| Aortic cross-clamp time | 133 (115–161) |
CABG, coronary artery bypass graft; CPB, cardiopulmonary bypass; PEARS, personalised external aortic root support; PFO, patent foramen ovale; VSRR, valve-sparing root replacement.
In two patients scheduled for PEARS, an intraoperative conversion to VSRR was performed as the aorta was deemed too fragile (n=1), or after coronary injury occurred (n=1). As patients are entered into the AVIATOR Database by completed procedure, we did not capture conversions during intended VSRR. Seven (4.4%) patients undergoing PEARS needed an intraoperative or postoperative intervention for coronary injury or impingement (table 3). Three patients underwent coronary artery bypass graft (CABG) for injury to the right coronary artery (two without consequences, one suffered a myocardial infarction). One patient underwent intraoperative CABG for refractory ventricular fibrillation. In one patient, the longitudinal seam of the ExoVasc was urgently reopened due to right ventricular stunning. One patient underwent intraoperative CABG for left ventricular failure after mitral valve repair with concomitant PEARS. The left main stem was injured in one patient with a severe pectus deformity, as previously reported.10 This patient, in whom the PEARS procedure was aborted, died 6 days postoperatively from intracranial bleeding while on extracorporeal membrane oxygenation, resulting in a 0.6% early mortality for PEARS. Among patients undergoing VSRR, there were no early deaths, and one patient underwent postoperative stenting of the posterior descending artery. Ten (7%) patients in the VSRR group underwent a reintervention for bleeding or tamponade, while there were none after PEARS (p<0.001). An overview of in-hospital outcomes is shown in table 3.
Table 3.
In-hospital and postoperative outcomes for total and matched population
| Unmatched patients | Propensity score-matched patients | |||||
| PEARS (n=159) | VSRR (n=142) | P value | PEARS (n=80) | VSRR (n=80) | P value | |
| In-hospital outcomes | ||||||
| Reoperation for bleeding | 0 (0%) | 10 (7%) | <0.001 | 0 (0%) | 6 (7.5%) | 0.03 |
| Coronary revascularisation | 7 (4.4%) | 1 (0.7%) | 0.07 | 1 (1.3%) | 0 (0%) | 1.00 |
| Stroke | 1 (0.6%) | 2 (1.4%) | 0.60 | 1 (1.3%) | 1 (1.3%) | 1.00 |
| Perioperative dissection | 1 (0.6%) | 1 (0.7%) | 1.00 | 1 (1.3%) | 0 (0%) | 1.00 |
| Perioperative death | 1 (0.6%) | 0 (0%) | 1.00 | 0 (0%) | 0 (0%) | – |
| Length of stay | 6 (5–7) | 7 (6–10) | <0.001 | 6 (5–7) | 7 (6–9) | <0.001 |
| AR grade postoperatively | <0.001 | 0.21 | ||||
| 0/4 | 144 (92.3%) | 100 (72.5%) | 69 (86.3%) | 62 (77.5%) | ||
| 1/4 | 12 (7.7%) | 34 (24.6%) | 9 (11.3%) | 16 (20.0%) | ||
| 2/4 | 0 (0%) | 4 (2.9%) | 0 (0%) | 0 (0%) | ||
| Postoperative outcomes | ||||||
| AV reintervention | 1 (0.6%) | 7 (4.9%) | 0.12 | 0 (0%) | 2 (2.5%) | 0.28 |
| AV/Asc/arch reintervention | 3 (1.9%) | 10 (7%) | 0.26 | 1 (1.3%) | 3 (3.8%) | 0.67 |
| Type A dissection | 0 (0%) | 0 (0%) | – | 0 (0%) | 0 (0%) | – |
| Type B dissection | 1 (0.6%) | 5 (3.5%) | 0.26 | 1 (1.3%) | 2 (2.5%) | 0.5 |
| Death | 2 (1.2%) | 4 (2.8%) | 0.96 | 1 (1.3%) | 3 (3.8%) | 0.45 |
| AR grade at last follow-up | <0.001 | <0.01 | ||||
| 0/4 | 129 (89.6%) | 64 (49.6%) | 64 (85.4%) | 41 (57.8%) | ||
| 1/4 | 14 (9.7%) | 51 (39.5%) | 10 (13.3%) | 25 (35.2%) | ||
| 2/4 | 1 (0.7%) | 4 (3.1%) | 1 (1.3%) | 2 (2.8%) | ||
| ≥3/4 | 0 (0%) | 10 (7.8%) | 0 (0%) | 3 (4.2%) | ||
Continuous variables compared using Mann-Whitney U test, categorical variables via χ2 or Fisher’s exact test. Matched data compared using Wilcoxon signed-rank test or McNemar’s test for related samples. When comparing Kaplan-Meier estimates, the log-rank test was used. Details on all reinterventions are shown in the online supplemental data.
AR, aortic regurgitation; Asc, ascending aorta; AV, aortic valve; PEARS, personalised external aortic root support; VSRR, valve-sparing root replacement.
heartjnl-2022-321840supp001.pdf (67KB, pdf)
Survival
Median follow-up duration for PEARS patients was 25 months (IQR 12–52, total of 542 postoperative patient years), while for VSRR patients, it was 55 months (IQR 23–89, 713 postoperative years). No follow-up after discharge could be collected for two (1.3%) PEARS and four (2.8%) VSRR patients. Overall survival at 5 years was similar for PEARS and VSRR at 95.8% vs 99.2% (p=0.27). In the matched cohorts, survival was also similar at 5 years: 98.3% vs 98.6% (p=0.99) for PEARS and VSRR, respectively. Underlying causes of death and details on postoperative complications are shown in online supplemental table S1.
Freedom from reintervention and aortic events
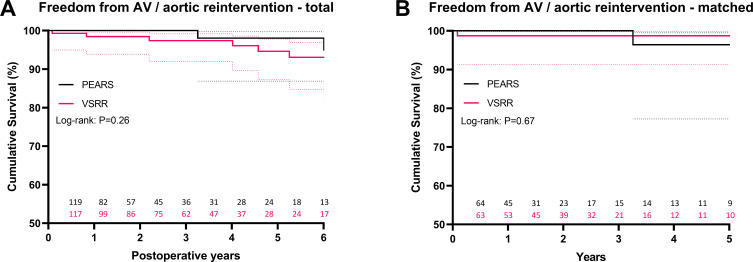
Freedom from valve, ascending aorta and arch reintervention at 5 years was similar with 98% for PEARS vs 94.6% for VSRR (p=0.1) (figure 2). All three reinterventions in the PEARS group were related to operator failure to achieve complete coverage by the PEARS mesh, with one patient needing a total root replacement at 6 years after PEARS and two cases of off-pump redo-PEARS at 3 and 9 years. Reinterventions in the VSRR group were related to AR and/or progression of distal aneurysmal disease, or mitral valve pathology (online supplemental table S1). Among the seven patients who underwent an aortic valve reintervention after VSRR, six were initially operated via the remodelling technique, with or without an external aortic annuloplasty. In the matched cohorts, freedom from aortic valve or aortic reintervention was also similar at 5 years: 96.4% for PEARS vs 98.7% for VSRR (p=0.89). There were no type A dissections in either group, while there were five (3.5%) type B dissections after VSRR as opposed to one (0.6%) after PEARS (p=0.26).
Figure 2.
Kaplan-Meier estimate of freedom from aortic valve (AV) and ascending aorta/arch reintervention for total (A) and matched (B) cohorts, including 95% CIs. Graphs were truncated when less than 15% of patients remained at risk. PEARS, personalised external aortic root support; VSRR, valve-sparing root replacement.
Aortic regurgitation
At last follow-up after PEARS (median 21 months, IQR 4–42), nearly 90% of patients had no AR and only one patient had an AR of 2/4. At last follow-up after VSRR (median 48 months, IQR 19–71), approximately 50% of patients had no AR while 10.9% had an AR of at least 2/4 (table 3).
Both preoperatively and at discharge, patients undergoing VSRR were significantly more likely to have mild (1/4) AR. After PS matching, there was no significant difference in AR grade at these time points (tables 1 and 3). Among matched PEARS patients, 85.4% had no AR and 14.6% had an AR grade of at least 1/4 at last follow-up (median 22 months, IQR 8–45). When comparing last echocardiographic follow-up of the matched PEARS group with intermediate follow-up after VSRR (median 22 months, IQR 5–35), patients who underwent VSRR had significantly higher AR grades: 61% had no AR and 39% had an AR grade of at least 1/4 (p=0.003).
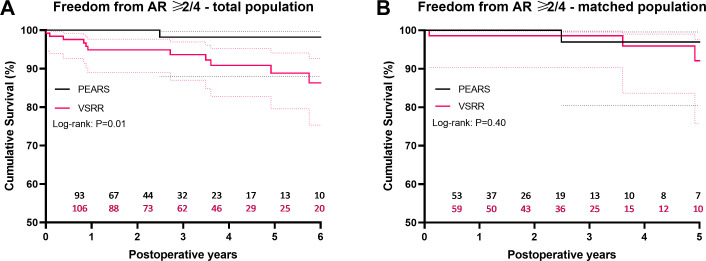
For the total population, freedom from AR ≥2/4 at 5 years was significantly greater after PEARS: 98.2% (n=23 at risk) vs 88.9% for VSRR (n=46 at risk) (p=0.02) (figure 3). In the matched population, there was no difference in freedom from AR ≥2/4 at 5 years (PEARS 97% vs VSRR 92.1%, p=0.55).
Figure 3.
Kaplan-Meier estimate of freedom AR ≥2/4 for the total (A) and matched (B) population, including 95% CIs. Graphs were truncated when less than 15% of patients remained at risk. AR, aortic regurgitation; PEARS, personalised external aortic root support; VSRR, valve-sparing root replacement.
Discussion
While the perioperative outcomes of PEARS and root replacement have been compared,11 this study represents the first side-by-side comparison of PEARS and VSRR evaluating safety, survival, freedom from reintervention and AR. Our observations fuel the relevant discussion on the challenges in referring and selecting patients with CTD for surgical treatment of their root aneurysm.12 In this subset of patients, both PEARS and VSRR aim to prevent ascending aortic dissection yet differ significantly in terms of surgical technique. PEARS is usually performed without use of cardiopulmonary bypass and preserves the blood–endothelial interface, while VSRR requires cardioplegic arrest and entails blood–prosthesis contact. PEARS is typically used in patients with at most mild AR and at a smaller root diameter than at which root replacement would be indicated.7
Our study cohort was derived from real-world clinical data and consists of a selected population of young patients with predominantly MFS, normal ejection fraction and at most mild AR undergoing elective surgery. The PEARS 200 Database represents the most extensive follow-up on PEARS to date, starting from patient 1 as the procedure was disseminated worldwide.7 The AVIATOR Initiative is an open international registry, including over 5000 patients by now, aiming to define the role of aortic valve repair in patients with AR and ascending aortic aneurysm.9 Our study needs to be interpreted in light of the challenges associated with comparing two retrospective databases with different duration of follow-up.
Patients undergoing VSRR were further along in their disease progression or had a more severe CTD phenotype than those undergoing PEARS. Most remarkable were differences in age, root diameter, EuroSCORE II and preoperative AR (table 1). Patients for whom the surgeon judged there was an indication for aortic root surgery at diameters below conventional thresholds may have additional risk factors, yet we did not have data on clinical decision-making available.1 The median aortic root diameter of 49 mm in our selected population of patients with MFS undergoing VSRR was similar to the diameter of 48–54 mm reported in earlier series, depending on selection criteria per centre.13 14 Among patients undergoing VSRR, 57% had no AR preoperatively and repair of at least one cusp was performed in 34.5%, most commonly via a central plicating stitch. Renowned centres report a highly selective approach to VSRR in MFS with 83%–85.6% of patients having at most mild AR and cusp repair performed in 10%–20%.13 15
With no perioperative deaths and a median EuroSCORE II of 1.6, VSRR was safe in this study, yet 7% of patients required a reintervention for bleeding. There was a 4.4% incidence of coronary complications observed with PEARS, including one death. This may be partially related to the learning curve of this emerging procedure, reflected by the fact that these events occurred early in the experience. On the other hand, this issue may not be wholly abolished as the procedure is technically challenging.
In this selected group of patients, both PEARS and VSRR seem to offer favourable and statistically similar mid-term survival and freedom from reintervention, both in the total and matched populations. While the reintervention rate after PEARS was 0.55% per year, the reintervention rate for VSRR was 1.4% per year, higher than the 0.6% per year found in a recent meta-analysis on the outcomes of root replacement in MFS.4 There was no difference in freedom from AR ≥2/4 at 5 years between matched groups, yet patients undergoing VSRR had significantly higher AR grades at last follow-up (table 3). While this could be related to longer follow-up in the VSRR group, the risk of developing AR is inherent to VSRR.16 In PEARS, aortic root geometry is preserved or slightly downscaled by a 95% luminal-diameter-scaled implant, likely improving leaflet coaptation.17 VSRR, on the other hand, entails a significant reduction of aortic root dimensions, thereby acutely altering leaflet configuration, potentially leading to cusp prolapse.16
The absence of type A dissections after PEARS in 159 patients with 542 years of follow-up indicates that this procedure has the potential to prevent aortic dissection, yet continued follow-up is needed. The elevated risk of type B dissection and reintervention, while not significant, in patients undergoing VSRR suggests that earlier intervention or more aggressive management of the distal aorta may be indicated. Furthermore, a stiff interposition graft as used in VSRR causes a marked increase in wall stress distally, while the PEARS mesh becomes incorporated histologically and has a gradual reduction in hoop strength from proximal to distal.18–21
There are several important limitations to our study. This retrospective, multicentre study used two different databases, including 301 patients operated at 33 centres between 1996 and 2021. While outcomes after PEARS were reported according to intention to treat, data were extracted from the AVIATOR Database per completed procedure. We could therefore not identify patients who underwent an intraoperative conversion during VSRR. Due to low event rates and differences in follow-up, our description of late outcomes was predominantly descriptive. While the outcomes of VSRR are influenced by procedure type (reimplantation and remodelling with or without external annuloplasty in this study) and patient selection, our study does not factor procedure type into the comparison with PEARS. As we did not have data on aortic valve morphology in the PEARS group, we were unable to compare patients accordingly. Furthermore, we only had data on one late echocardiography for PEARS patients while VSRR patients had more extensive follow-up. Our echocardiographic data should be interpreted in the setting of a multicentre study. Using a PS analysis, we aimed to quantify differences between patient populations and correct for selection bias between groups, yet, are unable to determine whether differences in AR grade after PEARS or VSRR are related to uncorrected confounders, different follow-up duration or the development of AR after VSRR. Using our matching approach, patients with outlying aortic root diameters may be excluded. We focused on variables indicative of disease severity while not including CTD phenotype in the PS model as we did not have data available on the underlying genetic mutations which are strongly related to disease severity.22
Conclusions
VSRR and PEARS both seem to offer favourable mid-term survival, freedom from reintervention and preservation of valve function in syndromic root aneurysm. Depending on the disease stage of the individual patient, both treatments may be complementary, yet long-term follow-up is needed for PEARS. Our study indicates that an earlier intervention in the disease progression via PEARS may be justified with a low probability of developing aortic events if the necessary attention goes to the coronary anatomy. Improved prediction of aortic complications is needed to help us guide patient selection. A shared decision-making process should involve weighing the risk of watchful waiting against the potential risks of all available surgical treatments.
heartjnl-2022-321840supp002.pdf (2.4MB, pdf)
Acknowledgments
We would like to thank the HVS AV Database team for their indispensable role in data collection and management. We would like to thank the following surgeons and centres who contributed patient data to the AVIATOR Database for this study: Monica Contino (Cardiovascular Surgery Department, Ospedale Luigi Sacco, Milan, Italy), Bardia Arabkhani (Department of Cardio-Thoracic Surgery, Leiden University Medical Center, Leiden, The Netherlands), José Aramendi (Department of Cardiac Surgery, Hospital de Cruces, Bilbao, Spain), Jan Vojacek (Department of Cardiac Surgery, University Hospital Hradec Kralove, Hradec Kralove, Czech Republic), Olivier Bouchot (Cardiac Surgery Department, Dijon University Hospital, Dijon, France), Jaroslav Hlubocky (Department of Cardiovascular Surgery, General University Hospital, Prague, Czech Republic), Ruggero De Paulis (Cardiac Surgery Department, European Hospital, Rome, Italy), Maciej Matuszewski (Cardiothoracic Surgery Department, New Cross Hospital, Wolverhampton, UK). We would like to thank the PEARS Project team and the following surgeons who contributed data on patients undergoing PEARS for this study: Conal Austin, Andreas Hoschtitzky, Ulrich Rosendahl, Mario Petrou, Fabio de Robertis, Massimo Caputo, Serban Stoica, Renzo Pessotto, Alastair Graham, Adam El Gamel, David Koolbergen, Mark Hazekamp, Jan Pirk, Ivo Skalski, Petr Kacer, Petr Santavy, Petr Nemec, Pavel Zacek, John Chan, Peter Tesar, Nelson Alphonso, Prem Venugopal. We would also like to thank those who assisted in data collection (Radka Kockova, Beverley Horne, Lydie Tauchenova, Katie O’Sullivan, Yvonne May, Jessica Suna, Ana Redondo). The authors would like to thank the radiologists at the Royal Brompton Hospital (Raad Mohiaddin, Cemil Izgi, Michael Rubens and Tom Semple). Finally, we would like to thank the team at Exstent for their dedication to manufacturing ExoVasc implants.
Footnotes
Collaborators: Lucas Van Hoof, Marie Lamberigts, Dries Noé, Bart Meuris, Filip Rega, Peter Verbrugghe (Department of Cardiac Surgery, University Hospitals Leuven, Leuven, Belgium). On behalf of the PEARS Project team: Tom Treasure (Clinical Operational Research Unit, University College London, London, UK), John Pepper (Royal Brompton and Harefield NHS Foundation Trust, London, UK). On behalf of the Aortic Valve Repair Research Network Investigators from the Heart Valve Society, in descending order of VSRR cases contributed to AVIATOR subpopulation: Ismail El-Hamamsy (Department of Cardiovascular Surgery, The Mount Sinai Hospital, New York, New York, USA/Montréal Heart Institute, Montreal, Canada), Emmanuel Lansac (Department of Cardiothoracic Surgery, Pitié-Salpêtrière University Hospital, Paris, France/Institut Mutualiste Montsouris, Paris, France), Jolanda Kluin (Department of Cardiothoracic Surgery, University Medical Centers, Amsterdam, The Netherlands), Laurent de Kerchove (Division of Cardiothoracic and Vascular Surgery, Cliniques Universitaires Saint-Luc, Brussels, Belgium).
Contributors: This study was designed by LVH, PV, ML, FR and BM. Data on patients undergoing PEARS were collected by LVH, TT, JP and FR in the framework of an earlier study published in Heart. JP performed the first 26 PEARS operations starting in 2004 and both TT and JP have been involved in the PEARS Project team since the conceptualisation of the procedure in 2000. IE-H, EL, JK and LdK were the leading surgeons in the centres contributing the majority of patients who underwent VSRR in the AVIATOR Database. Furthermore, they are leading members of the Heart Valve Society AV Database AVIATOR. Data analysis was performed by LVH, ML, DN, PV, FR and BM with input from all coauthors. The first draft of the manuscript was prepared by LVH, ML, DN, PV, FR and BM. All authors contributed to and approved the final version to be published. PV acts as guarantor.
Funding: LVH is supported by a Doctoral Grant Strategic Basic Research (SB 1S70220N) from the Research Foundation Flanders (FWO). This research project has been made possible thanks to the HVS AV Database data team, funded by Edwards Lifesciences.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
Collaborators: On behalf of the Aortic Valve Repair Research Network Investigators from the Heart Valve Society, Lucas Van Hoof, Marie Lamberigts, Dries Noé, Bart Meuris, Filip Rega, Peter Verbrugghe, Tom Treasure, John Pepper, Ismail El-Hamamsy, Emmanuel Lansac, Jolanda Kluin, and Laurent de Kerchove
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
For this retrospective study, the need for additional patient consent was waived (Ethical Committee of the University Hospitals Leuven, S63787).
References
- 1. Isselbacher EM, Preventza O, Hamilton Black J, et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease. J Am Coll Cardiol 2022;80:e223–393. 10.1016/j.jacc.2022.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Benedetto U, Melina G, Takkenberg JJM, et al. Surgical management of aortic root disease in Marfan syndrome: a systematic review and meta-analysis. Heart 2011;97:955–8. 10.1136/hrt.2010.210286 [DOI] [PubMed] [Google Scholar]
- 3. David TE, David CM, Manlhiot C, et al. Outcomes of aortic valve-sparing operations in marfan syndrome. J Am Coll Cardiol 2015;66:1445–53. 10.1016/j.jacc.2015.07.041 [DOI] [PubMed] [Google Scholar]
- 4. Flynn CD, Tian DH, Wilson-Smith A, et al. Systematic review and meta-analysis of surgical outcomes in Marfan patients undergoing aortic root surgery by composite-valve graft or valve sparing root replacement. Ann Cardiothorac Surg 2017;6:570–81. 10.21037/acs.2017.11.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pepper J, Golesworthy T, Utley M, et al. Manufacturing and placing a bespoke support for the Marfan aortic root: description of the method and technical results and status at one year for the first ten patients. Interact Cardiovasc Thorac Surg 2010;10:360–5. 10.1510/icvts.2009.220319 [DOI] [PubMed] [Google Scholar]
- 6. Treasure T, Takkenberg JJM, Golesworthy T, et al. Personalised external aortic root support (PEARS) in Marfan syndrome: analysis of 1-9 year outcomes by intention-to-treat in a cohort of the first 30 consecutive patients to receive a novel tissue and valve-conserving procedure, compared with the published results of aortic root replacement. Heart 2014;100:969–75. 10.1136/heartjnl-2013-304913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Van Hoof L, Rega F, Golesworthy T, et al. Personalised external aortic root support for elective treatment of aortic root dilation in 200 patients. Heart 2021;107:1790–5. 10.1136/heartjnl-2021-319300 [DOI] [PubMed] [Google Scholar]
- 8. Nienaber CA, Yuan X, Ernst S. PEARS procedure and the difficulty to provide evidence for its benefits. Eur Heart J 2020;41:4086–8. 10.1093/eurheartj/ehaa596 [DOI] [PubMed] [Google Scholar]
- 9. de Heer F, Kluin J, Elkhoury G, et al. Aviator: an open international registry to evaluate medical and surgical outcomes of aortic valve insufficiency and ascending aorta aneurysm. J Thorac Cardiovasc Surg 2019;157:2202–11. 10.1016/j.jtcvs.2018.10.076 [DOI] [PubMed] [Google Scholar]
- 10. Treasure T, Petrou M, Rosendahl U, et al. Personalized external aortic root support: a review of the current status. Eur J Cardiothorac Surg 2016;50:400–4. 10.1093/ejcts/ezw078 [DOI] [PubMed] [Google Scholar]
- 11. Treasure T, Crowe S, Chan KMJ, et al. A method for early evaluation of a recently introduced technology by deriving a comparative group from existing clinical data: a case study in external support of the Marfan aortic root. BMJ Open 2012;2:e000725–10. 10.1136/bmjopen-2011-000725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gökalp AL, Takkenberg JJM. Decision-making in thoracic aortic aneurysm surgery-clinician and patient view. Semin Thorac Cardiovasc Surg 2019;31:638–42. 10.1053/j.semtcvs.2019.05.032 [DOI] [PubMed] [Google Scholar]
- 13. Price J, Magruder JT, Young A, et al. Long-term outcomes of aortic root operations for Marfan syndrome: a comparison of Bentall versus aortic valve-sparing procedures. J Thorac Cardiovasc Surg 2016;151:330–8. 10.1016/j.jtcvs.2015.10.068 [DOI] [PubMed] [Google Scholar]
- 14. Schoenhoff FS, Langhammer B, Wustmann K, et al. Decision-making in aortic root surgery in Marfan syndrome: bleeding, thromboembolism and risk of reintervention after valve-sparing or mechanical aortic root replacement. Eur J Cardiothorac Surg 2015;48:931–6. 10.1093/ejcts/ezu553 [DOI] [PubMed] [Google Scholar]
- 15. Coselli JS, Volguina IV, LeMaire SA, et al. Midterm outcomes of aortic root surgery in patients with Marfan syndrome: a prospective, multicenter, comparative study. J Thorac Cardiovasc Surg 2021. doi: 10.1016/j.jtcvs.2021.08.064. [Epub ahead of print: 04 Sep 2021]. [DOI] [PubMed] [Google Scholar]
- 16. Lansac E, de Kerchove L. Aortic valve repair techniques: state of the art. Eur J Cardio-thoracic Surg 2018;53:1101–7. 10.1093/ejcts/ezy176 [DOI] [PubMed] [Google Scholar]
- 17. Izgi C, Newsome S, Alpendurada F, et al. External aortic root support to prevent aortic dilatation in patients with marfan syndrome. J Am Coll Cardiol 2018;72:1095–105. 10.1016/j.jacc.2018.06.053 [DOI] [PubMed] [Google Scholar]
- 18. Jayendiran R, Nour B, Ruimi A. A fluid–structure interaction analysis of anisotropic Dacron fabric used for aortic replacement. J Fluids Struct 2020;97:103108. 10.1016/j.jfluidstructs.2020.103108 [DOI] [Google Scholar]
- 19. Galea N, Piatti F, Lau C, et al. 4D flow characterization of aortic blood flow after valve sparing root reimplantation procedure. J Vis Surg 2018;4:95. 10.21037/jovs.2018.03.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pepper J, Goddard M, Mohiaddin R, et al. Histology of a marfan aorta 4.5 years after personalized external aortic root support. Eur J Cardio-Thoracic Surg 2015;48:502–5. 10.1093/ejcts/ezu415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vanderveken E, Vastmans J, Claus P, et al. Mechano-biological adaptation of the pulmonary artery exposed to systemic conditions. Sci Rep 2020;10:1–12. 10.1038/s41598-020-59554-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Regalado ES, Morris SA, Braverman AC, et al. Comparative risks of initial aortic events associated with genetic thoracic aortic disease. J Am Coll Cardiol 2022;80:857–69. 10.1016/j.jacc.2022.05.054 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
heartjnl-2022-321840supp001.pdf (67KB, pdf)
heartjnl-2022-321840supp002.pdf (2.4MB, pdf)
Data Availability Statement
Data are available upon reasonable request.