Abstract
Background
Interhospital transfer of stroke patients (drip and ship concept) is associated with longer treatment times compared with primary admission to a comprehensive stroke center (mothership concept). In recent years, studies on a novel concept of performing endovascular thrombectomy (EVT) at external hospitals (EXT) by transferring neurointerventionalists, instead of patients, have been published. This collaborative study aimed at answering the question of whether EXT saves time in the workflow of acute stroke treatment across various geographical regions.
Methods
This was a patient level pooled analysis of one prospective observational study and four retrospective cohort studies, the EVEREST collaboration (EndoVascular thrombEctomy at Referring and External STroke centers). Time from initial stroke imaging to EVT (vascular puncture) was compared in mothership, drip and ship, and EXT concepts.
Results
In total, 1001 stroke patients from various geographical regions who underwent EVT due to large vessel occlusion were included. These were divided into mothership (n=162, 16.2%), drip and ship (n=458, 45.8%), and EXT (n=381, 38.1%) cohorts. The median time periods from onset to EVT (195 min vs 320 min, p<0.001) and from imaging to EVT (97 min vs 184 min, p<0.001) in EXT were significantly shorter than for drip and ship thrombectomy concept.
Conclusions
This pooled analysis of the EVEREST collaboration adds evidence that performing EVT at external hospitals can save time compared with drip and ship across various geographical regions. We encourage conducting randomized controlled trials comparing both triage concepts.
Keywords: Stroke, Thrombectomy
Introduction
Several randomized controlled trials have shown that endovascular thrombectomy (EVT) is an effective treatment for large vessel occlusions.1 As stated in the stroke guidelines by the American Heart Association and the American Stroke Association, this treatment requires the stroke patient to be at an experienced stroke center with rapid access to cerebral angiography, qualified neurointerventionalists, and a comprehensive periprocedural care team.2 Area-wide coverage of this high level of expertise, especially the availability of neurointerventionalists, remains variable.3 A European survey of national scientific societies and stroke experts, for example, showed major inequalities in access to EVT between and within European countries.4
In most regional stroke networks there are two established triage concepts for EVT: first, stroke patients may be directly admitted to a comprehensive stroke center (CSC) (mothership (MS)), if this is the closest treatment option. Second, if the closest CSC is further away, stroke patients may be transported to the closest primary stroke center in order to avoid a delay of intravenous thrombolysis.5–7 In the case of a large vessel occlusion, these patients are secondarily transferred to a CSC (drip and ship (DS)).
However, there are studies suggesting a worse outcome for DS patients compared with MS patients, secondary to time delays.8–11 Some of these DS hospitals are sufficiently equipped with CT, MRI, an angiography suite, and stroke neurologists, as well as a stroke unit and an intensive care unit for postprocedural care. Generally, these hospitals are capable of performing EVT (thrombectomy capable stroke centers (TSCs)). They only lack a team of neurointerventionalists. Indeed, there is a lack of neurointerventionalists in acute stroke care, as reported by Bulwa and Chen.12
Since these TSCs have difficulties hiring neurointerventionalists, agreements between CSCs and TSCs in some stroke networks have been established. Instead of transferring a stroke patient with a large vessel occlusion from a TSC to a CSC, a neurointerventionalist is transferred to perform a thrombectomy at external hospitals (TSCs, EXT concept). The idea behind this triage option is that time can be saved in the acute treatment phase. Depending on the healthcare and reimbursement system, there might also be a financial benefit for the TSC. In addition, this concept may reduce resource use from emergency medical services and stroke units of CSCs.
The results of this triage concept have been published under varying names, such as drip and drive, trip and treat, Mobile Interventional Stroke Team model, drive the doctor, and drive and retrieve.13–20 These studies have demonstrated that EXT can save time compared with DS in those geographical regions.21 However, the EXT concept has not been considered in national or international stroke guidelines.2 22
The purpose of this study was to pool patient data from five studies and to compare time metrics from initial stroke imaging to the initiation of EVT in DS and EXT in a large scale analysis. We hypothesize that EXT can save time compared with DS across various geographical regions.
Methods
Study design and setting
The EVEREST (EndoVascular thrombEctomy at Referring and External STroke centers) collaborative group was established to compare the EXT concept with DS across various geographical regions. Four neurointerventional departments participated in this collaboration: Department of Neurosurgery, Icahn School of Medicine, Mount Sinai, New York City; Department of Neuroradiology, Heidelberg University; Department of Neurosurgery, Hokkaido University; and Department of Neuroradiology, University of Hamburg-Eppendorf. These departments provide EVT at their CSCs and at TSCs in metropolitan and/or rural areas. Details on these stroke networks are provided in the online supplemental file. These centers have published data on the EXT concept and compared this concept with DS and/or MS.13 14 17 19 23 Data from these published studies were pooled for an analysis (table 1). Hokkaido University provided additional patients outside of their study. Details on each study are provided in the online supplemental material.
Table 1.
Studies included in this pooled analysis
| Study, No of patients | Stroke networks | Triage concepts | Years of recruiting | Year published |
| Seker, n=12615 | Heidelberg, Germany | DS, EXT | 2012–16 | 2018 |
| Brekenfeld, n=7413 | Hamburg, Germany | DS, EXT | 2016 | 2018 |
| Osanai, n=133*14 | Hokkaido, Japan | EXT | 2015–19 | 2019 |
| NEUROSQUAD, n=44017 | Heidelberg, Germany and Hamburg, Germany | DS, EXT, MS | 2018 | 2020 |
| Morey, n=22819 | New York City, USA | DS, EXT, MS | 2016–18 | 2020 |
*67 patients were included from the initial study, 66 patients were treated after publication of this study until December 2019.14
DS, drip and ship; EXT, thrombectomy at external hospital; MS, mothership.
neurintsurg-2021-018049supp001.pdf (21.8MB, pdf)
All data were deidentified and unlinked to any identifiers. The pseudonymized data of each study were sent to one author (FS) for statistical analysis. The institutional review board of each participating center approved the study and waived the need for patient consent. This manuscript was written according to the Strengthening the Reporting of Observational Studies in Epidemiology guidelines.
Study cohort
All patients in the pooled analysis underwent thrombectomy and were treated according to national guidelines and the inhouse standards of each participating site. Intravenous thrombolysis was given according to national guidelines that were valid during the time of treatment. The occlusion sites were defined according to catheter angiography.
Patients in the included studies were pooled and classified as follows: DS, if the patient was transported to a primary stroke center first and secondarily transferred to a CSC for thrombectomy; and EXT, if the patient was transported to a TSC without on-site neurointerventionalists and thrombectomy was performed by a neurointerventionalist who was transferred from a CSC to this hospital. For comparison reasons, MS patients, that is, direct transport to a CSC with on-site neurointerventionalists, were also included. The main focus of this study was the comparison of DS and EXT.
Outcome measures
The primary outcome measure was the time from stroke imaging (acquisition of the first image) to the start of EVT (ie, vascular puncture). Secondary outcome measures were time from onset or last seen well to initial stroke imaging and time from onset or last seen well to the start of EVT (ie, vascular puncture).
Statistical analysis
Statistical analysis was performed using R V.3.6.2 and RStudio V.1.2.5033. Quantitative variables are expressed as means (SD) or median (IQR). Categorical variables are expressed as numbers (percentages). MS, DS, and EXT were compared using a Kruskal–Wallis test. The Conover test with Benjamini–Hochberg correction was used for post hoc group comparison. A p value <0.05 was considered statistically significant.
Results
In total, 1001 patients were included in this study. At one stroke network, data from three TSCs (n=53) were excluded because these TSCs left the network. This stroke network also excluded data from 10 MS and DS patients and has only provided data on EXT patients.
Of these 1001 patients, 162 (16.2%) were in the MS group, 458 (45.8%) were in the DS group, and 381 (38.1%) were in the EXT group.
Age, sex, stroke severity, and occlusion sites were similar among the groups. Intravenous thrombolysis was given less frequently in the MS group (MS 34% vs DS 58.3% and EXT 59.1%, p<0.001) (table 2).
Table 2.
Demographics
| MS (n=162) | DS (n=458) | EXT (n=381) | P value | |
| Age (years) (mean (SD)) | 72.1 (12.4) | 71.9 (13.8) | 74.1 (12.7) | 0.074 |
| Women (n (%)) | 82 (50.6) | 229 (50.0) | 201 (52.8) | 0.721 |
| Baseline NIHSS (median (IQR)) | 15 (9–21) | 16 (12–20) | 16 (12–20) | 0.361 |
| Occlusion site (n (%)) | ||||
| ICA | 34 (21.0) | 114 (24.9) | 109 (28.6) | 0.483 |
| MCA | 113 (69.8) | 301 (65.7) | 242 (63.5) | |
| BA | 14 (7.4) | 33 (7.2) | 23 (6.0) | |
| Other | 3 (1.9) | 10 (2.1) | 7 (1.8) | |
| Intravenous thrombolysis (n (%)) | 55 (34.0) | 267 (58.3) | 225 (59.1) | <0.001 |
BA, basilar artery; DS, drip and ship; EXT, thrombectomy at external hospital; ICA, internal carotid artery; MCA, middle cerebral artery; MS, mothership; NIHSS, National Institutes of Health Stroke Scale.
Median time from imaging to thrombectomy was the shortest in the MS cohort (56 min). It was significantly shorter in the EXT cohort compared with the DS cohort (97 vs 184 min, p<0.001) (table 3, figure 1).
Table 3.
Comparison of time metrics in the three triage concepts
| MS (n=162, 16.2%) | DS (n=458, 45.8%) | EXT (n=381, 38.1%) | P value* | P value† MS vs DS |
P value† MS vs EXT |
P value† DS vs EXT |
|
| Onset to imaging (min) | 110 (69–313) | 113 (65–265) | 93 (57–171) | <0.001 | 0.742 | 0.002 | <0.001 |
| Onset to EVT (min) | 180 (130–422) | 320 (243–480) | 195 (145–274) | <0.001 | <0.001 | 0.27 | <0.001 |
| Imaging to EVT (min) | 56 (42–80) | 184 (145–225) | 97 (53–130) | <0.001 | <0.001 | <0.001 | <0.001 |
Time metrics given as median (IQR).
*Kruskal–Wallis test between MS, DS, and EXT.
†Post hoc Conover test with Benjamini–Hochberg correction.
DS, drip and ship; EVT, endovascular thrombectomy; EXT, thrombectomy at external hospital; MS, mothership.
Figure 1.
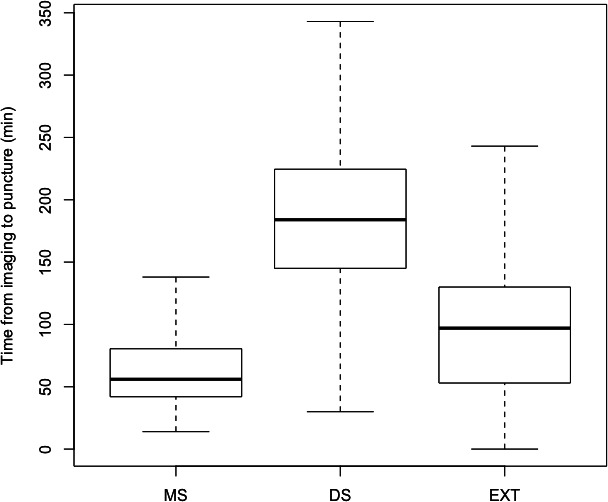
Box plot demonstrating time from stroke imaging to thrombectomy (vascular puncture) in the three cohorts: mothership (MS), drip and ship (DS), and thrombectomy at external hospital (EXT).
Median time from onset to imaging was shorter in the EXT cohort (93 min) compared with the MS (110 min, p=0.002) and DS cohorts (113 min, p<0.001). Median time from onset to thrombectomy was shorter in the EXT cohort (195 min) compared with the DS cohort (320 min, p<0.001) and similar to the MS cohort (180 min, p=0.27).
Discussion
The triage of stroke patients with large vessel occlusion has been of increasing interest in recent years.12 It is well established that the transfer of stroke patients from primary stroke centers to a CSC is time consuming, given the required coordination of interhospital transport and patient preparation for the transfer.6 17 21 24–27 The degree to which these activities prolong time to treatment are even more apparent for patients living in rural areas.9
The time delay present in the DS concept led to the idea of transferring neurointerventionalists from CSCs to perform EVT at external hospitals, which are thrombectomy capable (EXT concept). Eventually, compared with a patient, a neurointerventionalist can be transferred more easily to another hospital (eg, by driving or by taking a taxi). In a metro area such as New York City, even public transport can be faster than an ambulance.
Several retrospective single center studies, one prospective single center study, and one prospective bicenter study from the USA, Germany, and Japan have been published to date.13–15 17 19 All of these studies reported that the EXT concept (under a variety of names) can save time in the acute phase in their respective geography. However, the logistics of these stroke networks are different. In the EVEREST collaboration, we have pooled our data to compare DS and EXT in a broader spectrum. To avoid confusion, we have chosen to use the abbreviation EXT (thrombectomy at external hospitals) for this triage concept in this study.
In this pooled analysis with 1001 patients, we showed that the EXT concept was time saving across various geographical regions. This concept has been implemented successfully in both metropolitan areas, New York City and Hamburg, that regularly deal with traffic congestion, and in rural areas around Hokkaido and Heidelberg. The results of this pooled analysis, therefore, confirm the previous publications of each individual stroke network, which were mostly single center analyses. This study adds evidence that the EXT concept may be a good alternative to the well established DS concept if certain requirements are met.28 The EVEREST collaboration therefore encourages stroke networks to consider the EXT concept as a third triage pathway.29
The advantage of the EXT concept is that processes can be parallelized: during the transfer of the neurointerventionalist, the patient can be prepared in the angiography suite. This might explain why the time from imaging to thrombectomy was significantly shorter in the EXT cohort compared with the DS cohort. Some stroke networks use helicopters to transfer neurointerventionalists which may be even more time saving.30 31 However, availability of helicopters is limited and not every neurointerventionalist might be willing to fly in a helicopter.
Despite the advantages of the EXT concept, there are several issues to consider. This triage concept is demanding in terms of human resources.32 It requires at least two neurointerventionalists to be on call at the CSC, as there might be, for example, two simultaneous EVT cases. While one neurointerventionalist is treating a stroke patient at an external hospital, another neurointerventionalist needs to cover the CSC in the meantime. Therefore, not every CSC is capable of providing 24/7 EVT services for TSCs. In principal, it would be reasonable to employ one neurointerventionalist at a TSC to secure an EVT service. However, adding more neurointerventionalists at the CSC may be easier and more efficient than converting TSCs to CSCs because neurointerventionalists are generally more interested in working at a CSC due to the variety of neurointerventional cases and working in a larger and specialized team compared with a TSC. This may make TSCs less attractive for neurointerventionalists. Indeed, many TSCs have trouble finding neurointerventionalists willing to work under these difficult conditions, such as mostly working alone and not in a team, being solely responsible for EVT calls, having no back up in difficult cases, and no structured training. Therefore, converting TSCs to CSCs may not be a realistic option in many stroke networks.
Further concerns have been raised regarding the EXT concept.12 28 Not every primary stroke center is qualified to be a TSC. We agree that TSCs need to be adequately equipped and staffed. It is not enough that TSCs are capable of performing EVT, but they need to be excellent, as correctly pointed out by Mack et al in the editor’s column of JNIS.28 Physicians performing EVT procedures at TSCs need to have sufficient neurointerventional training. Since the EXT concept is a team effort and relies not only on neurointerventionalists, but neurologists, anesthesiologists, nurses, and radiographers at these external hospitals also need to be trained to optimize the workflow. Furthermore, quality of care at TSCs needs to be adequately monitored.
Recent papers may have created the impression that there is an agenda of converting as many primary stroke centers as possible to TSCs and performing EVT at TSCs rather than at CSCs.12 We would like to point out that this is not our intention. Offering EVT services at external hospitals is demanding in many ways, as explained above. EVT should only be performed at TSCs, if reasonable. This decision should be left to the discretion of the neurointerventional team and not be decided by hospital administrations or governmental institutions.
The strengths of this patient level pooled analysis are the large number of patients and its real world application. The main limitations of this study are its observational design and data selection from single centers. It cannot be excluded that structural advantages or disadvantages in certain regions may have influenced the results. Also, there might be selection bias, as patients were not selected consecutively. Each stroke network participating in this pooled analysis has published studies on the EXT concept with different study sizes. Hence the number of patients in this pooled analysis was not balanced among the stroke networks as this was not an a priori planned multicenter trial. For example, one of the four stroke networks provided about 40% of the data. Also, the time periods in which the patients were included might have varied. Therefore, some stroke networks might be underrepresented, which might have led to bias. The idea behind the DS concept is to reduce the time from onset to imaging for patients in rural areas. In our study, however, onset to imaging time was longer in DS compared with MS and EXT. This might be due to various reasons, such as geographical or logistical circumstances. Also, intravenous thrombolysis was less frequently given in the MS group (ie, the number of patients with contraindications for thrombolysis was presumably higher in this group). A possible explanation is that in these cases, emergency medical services may have decided to bypass primary stroke centers and go directly to the next CSC. Interestingly, the results of the recent RACECAT trial suggested that patients with certain prehospital clinical criteria bypass a primary stroke center if the next CSC is less than 30 min driving distance from the primary stroke center.33
Conclusion
This pooled analysis adds evidence that performing thrombectomy in external hospitals (EXT concept) can save time compared with the drip and ship concept in both rural and metropolitan areas across various geographical regions.
neurintsurg-2021-018049supp002.pdf (359.2KB, pdf)
Footnotes
Twitter: @moreyjr917, @Fie0815
Contributors: All authors made substantial contributions to the conception, design, data acquisition, data analysis, and data interpretation. FS drafted the manuscript and all other authors revised it critically and made substantial contributions. MAM acted as the guarantor. All authors approved the final version to be published. They agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the manuscript are appropriately investigated and resolved.
Funding: Two studies included in this collaborative pooled analysis were funded by Stryker.
Map disclaimer: The inclusion of any map (including the depiction of any boundaries therein), or of any geographic or locational reference, does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Competing interests: FS: research support from Stryker and member of the editorial board of JNIS. JF: research support from German Ministry of Science and Education (BMBF), German Ministry of Economy and Innovation (BMWi), German Research Foundation (DFG), European Union (EU), Hamburgische Investitions-und Förderbank (IFB), Medtronic, Microvention, Philips, and Stryker; consultant for Acandis, Boehringer Ingelheim, Cerenovus, Covidien, Evasc Neurovascular, MD Clinicals, Medtronic, Medina, Microvention, Penumbra, Route92, Stryker, and Transverse Medical; and member of the editorial board of JNIS. MAM: unrelated: board membership of Codman; consultancy for Medtronic, MicroVention, and Stryker; grants/grants pending from Balt, and MicroVention (money paid to the institution); payment for lectures including service on speakers bureaus for Medtronic, MicroVention, and Stryker. MB: (all unrelated): research support from Stryker, European Union, DFG, Hopp Foundation, Novartis, and Siemens; consultancy for Vascular Dynamics, Boehringer, and BBraun; personal fees from Novartis, Grifols, Merck, TEVA and Bayer.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by Heidelberg University Ethics Committee (S-462/2016). The ethics committee waived informed consent.
References
- 1. Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016;387:1723–31. 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 2. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2019;50:e344–418. 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 3. Weber R, Bartig D, Krogias C, et al. Letter to the editor: analysis of stroke patient migration for mechanical thrombectomy and changes in neurointerventional center size in Germany. Neurol Res Pract 2021;3:32. 10.1186/s42466-021-00131-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aguiar de Sousa D, von Martial R, Abilleira S, et al. Access to and delivery of acute ischaemic stroke treatments: a survey of national scientific societies and stroke experts in 44 European countries. Eur Stroke J 2019;4:13–28. 10.1177/2396987318786023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jauch EC, Schwamm LH, Panagos PD, et al. Recommendations for regional stroke destination plans in rural, suburban, and urban communities from the prehospital stroke system of care consensus conference: a consensus statement from the American Academy of Neurology, American Heart Association/American Stroke Association, American Society of Neuroradiology, National Association of EMS Physicians, National Association of State EMS Officials, Society of NeuroInterventional Surgery, and Society of Vascular and Interventional Neurology: endorsed by the Neurocritical Care Society. Stroke 2021;52:e133–52. 10.1161/STROKEAHA.120.033228 [DOI] [PubMed] [Google Scholar]
- 6. Seker F, Bonekamp S, Rode S. Direct admission vs. secondary transfer to a comprehensive stroke center for thrombectomy: retrospective analysis of a regional stroke registry with 2797 patients. Clin Neuroradiol 2019. [DOI] [PubMed] [Google Scholar]
- 7. Seker F, Bonekamp S, Rode S, et al. Impact of bridging thrombolysis on clinical outcome in stroke patients undergoing endovascular thrombectomy: a retrospective analysis of a regional stroke registry. Neuroradiology 2021;63:935–41. 10.1007/s00234-020-02619-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ismail M, Armoiry X, Tau N, et al. Mothership versus drip and ship for thrombectomy in patients who had an acute stroke: a systematic review and meta-analysis. J Neurointerv Surg 2019;11:14–19. 10.1136/neurintsurg-2018-014249 [DOI] [PubMed] [Google Scholar]
- 9. Pérez de la Ossa N, Abilleira S, Dorado L, et al. Access to endovascular treatment in remote areas. Stroke 2016;47:1381–4. 10.1161/STROKEAHA.116.013069 [DOI] [PubMed] [Google Scholar]
- 10. Rinaldo L, Brinjikji W, McCutcheon BA, et al. Hospital transfer associated with increased mortality after endovascular revascularization for acute ischemic stroke. J Neurointerv Surg 2017;9:1166–72. 10.1136/neurintsurg-2016-012824 [DOI] [PubMed] [Google Scholar]
- 11. Ali SF, Fonarow G, Liang L, et al. Rates, characteristics, and outcomes of patients transferred to specialized stroke centers for advanced care. Circ Cardiovasc Qual Outcomes 2018;11:e003359. 10.1161/CIRCOUTCOMES.116.003359 [DOI] [PubMed] [Google Scholar]
- 12. Bulwa Z, Chen M. Stroke center designations, neurointerventionalist demand, and the finances of stroke thrombectomy in the United States. Neurology 2021;97:S17–24. 10.1212/WNL.0000000000012780 [DOI] [PubMed] [Google Scholar]
- 13. Brekenfeld C, Goebell E, Schmidt H, et al. ‘Drip-and-drive’: shipping the neurointerventionalist to provide mechanical thrombectomy in primary stroke centers. J Neurointerv Surg 2018;10:932–6. 10.1136/neurintsurg-2017-013634 [DOI] [PubMed] [Google Scholar]
- 14. Osanai T, Ito Y, Ushikoshi S, et al. Efficacy of ‘drive and retrieve’ as a cooperative method for prompt endovascular treatment for acute ischemic stroke. J Neurointerv Surg 2019;11:757–61. 10.1136/neurintsurg-2018-014296 [DOI] [PubMed] [Google Scholar]
- 15. Seker F, Möhlenbruch MA, Nagel S, et al. Clinical results of a new concept of neurothrombectomy coverage at a remote hospital-"drive the doctor". Int J Stroke 2018;13:696–9. 10.1177/1747493018765267 [DOI] [PubMed] [Google Scholar]
- 16. Fiehler J. Direkt in ein neurovaskuläres Zentrum oder „drip and ship“? Radiology 2019;59:610–5. [DOI] [PubMed] [Google Scholar]
- 17. Seker F, Fiehler J, Möhlenbruch MA, et al. Time metrics to endovascular thrombectomy in 3 triage concepts: a prospective, observational study (NEUROSQUAD). Stroke 2020;51:335–7. 10.1161/STROKEAHA.119.027050 [DOI] [PubMed] [Google Scholar]
- 18. Wei D, Oxley TJ, Nistal DA, et al. Mobile interventional stroke teams lead to faster treatment times for thrombectomy in large vessel occlusion. Stroke 2017;48:3295–300. 10.1161/STROKEAHA.117.018149 [DOI] [PubMed] [Google Scholar]
- 19. Morey JR, Oxley TJ, Wei D, et al. Mobile interventional stroke team model improves early outcomes in large vessel occlusion stroke: the NYC mist trial. Stroke 2020;51:3495–503. 10.1161/STROKEAHA.120.030248 [DOI] [PubMed] [Google Scholar]
- 20. Morey JR, Zhang X, Marayati NF, et al. Mobile interventional stroke teams improve outcomes in the early time window for large vessel occlusion stroke. Stroke 2021;52:e527–30. 10.1161/STROKEAHA.121.034222 [DOI] [PubMed] [Google Scholar]
- 21. McTaggart RA, Holodinsky JK, Ospel JM, et al. Leaving no large vessel occlusion stroke behind. Stroke 2020;51:1951–60. 10.1161/STROKEAHA.119.026735 [DOI] [PubMed] [Google Scholar]
- 22. Turc G, Bhogal P, Fischer U, et al. European Stroke Organisation (ESO) - European Society for Minimally Invasive Neurological Therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischaemic stroke. Endorsed by Stroke Alliance for Europe (SAFE). Eur Stroke J 2019;4:6–12. 10.1177/2396987319832140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fatih S, Jens F, Möhlenbruch Markus A. Clinical outcome after endovascular thrombectomy in 3 triage concepts. Stroke:STROKEAHA.120.030520. [DOI] [PubMed] [Google Scholar]
- 24. Mokin M, Gupta R, Guerrero WR, et al. Aspects decay during inter-facility transfer in patients with large vessel occlusion strokes. J Neurointerv Surg 2017;9:442–4. 10.1136/neurintsurg-2016-012331 [DOI] [PubMed] [Google Scholar]
- 25. Park M-S, Yoon W, Kim J-T, et al. Drip, ship, and on-demand endovascular therapy for acute ischemic stroke. PLoS One 2016;11:e0150668. 10.1371/journal.pone.0150668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhao W, Ma P, Chen J, et al. Direct admission versus secondary transfer for acute ischemic stroke patients treated with thrombectomy: a systematic review and meta-analysis. J Neurol 2021;268:3601–9. 10.1007/s00415-020-09877-2 [DOI] [PubMed] [Google Scholar]
- 27. FC N, Low E, Andrew E. Deconstruction of interhospital transfer workflow in large vessel occlusion. Stroke 2017:STROKEAHA.117.017235. [DOI] [PubMed] [Google Scholar]
- 28. Mack WJ, Mocco J, Hirsch JA, et al. Thrombectomy stroke centers: the current threat to regionalizing stroke care. J Neurointerv Surg 2018;10:99–101. 10.1136/neurintsurg-2017-013721 [DOI] [PubMed] [Google Scholar]
- 29. Southerland AM, Park MS, Switzer JA, Switzer Jeffrey A. Thinking outside the mothership. Stroke 2020;51:3476–8. 10.1161/STROKEAHA.120.032633 [DOI] [PubMed] [Google Scholar]
- 30. Holodinsky JK, Williamson TS, Kamal N, et al. Drip and ship versus direct to comprehensive stroke center. Stroke 2017;48:233–8. 10.1161/STROKEAHA.116.014306 [DOI] [PubMed] [Google Scholar]
- 31. Hubert GJ, Kraus F, Maegerlein C. The “Flying Intervention Team”: a novel stroke care concept for rural areas. Cerebrovasc Dis 2021:1–8. [DOI] [PubMed] [Google Scholar]
- 32. Fargen KM, Arthur AS, Leslie-Mazwi T, et al. A survey of burnout and professional satisfaction among United States neurointerventionalists. J Neurointerv Surg 2019;11:1100–4. 10.1136/neurintsurg-2019-014833 [DOI] [PubMed] [Google Scholar]
- 33. Garcia-tornel Garcia A, Rubiera M, Olive-gadea M, et al. Abstract 26: timing the optimal transfer modality for suspected large-vessel stroke patients: a post-hoc analysis of the Racecat trial. Stroke 2022;53 10.1161/str.53.suppl_1.26 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
neurintsurg-2021-018049supp001.pdf (21.8MB, pdf)
neurintsurg-2021-018049supp002.pdf (359.2KB, pdf)
Data Availability Statement
No data are available.


