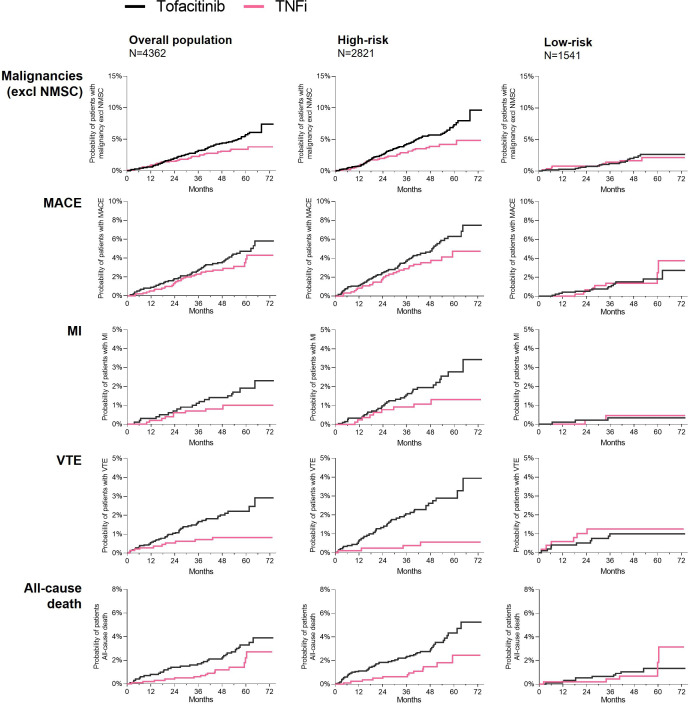
Figure 3.
Cumulative probability of patients with malignancies (excluding NMSC), MACE, MI, VTE and all-cause death in ORAL Surveillance overall population and by subgroups of high-risk and low-risk patients. Overall population received tofacitinib 5 mg or 10 mg two times a day (N=2911) or TNFi (N=1451). High-risk patients were ≥65 years of age or ever smoker (tofacitinib, N=1895; TNFi, N=926). Low-risk patients were <65 years of age and never smoker (tofacitinib, N=1016; TNFi, N=525). Cumulative probabilities of events were calculated based on Kaplan-Meier estimates. Cumulative probability plots for malignancies (excluding NMSC) and MACE in overall population have been reported in Ytterberg et al 1 and are included for reference. MACE, major adverse cardiovascular events; MI, myocardial infarction; NMSC, non-melanoma skin cancer; TNFi, tumour necrosis factor inhibitor; VTE, venous thromboembolism.