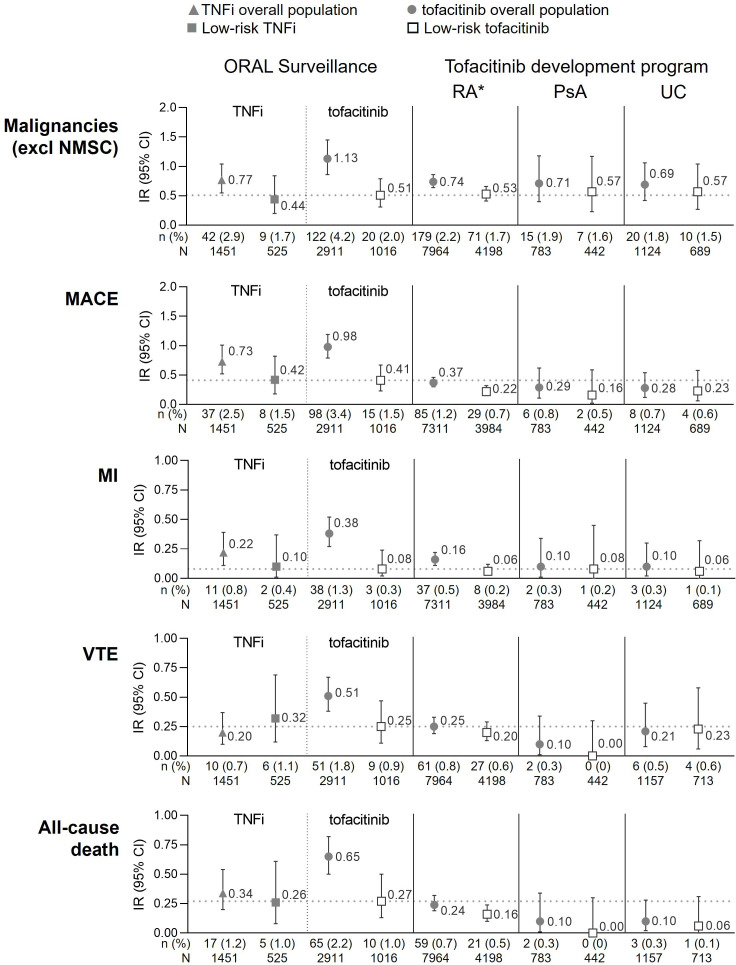
Figure 4.
Risk of malignancies (excluding NMSC), MACE, MI, VTE and all-cause death in low-risk populations in ORAL Surveillance and tofacitinib clinical development programmes. Low-risk patients were <65 years of age and never smoker. Horizontal dotted line represents IR in low-risk patients treated with tofacitinib in ORAL Surveillance. IRs express the number of patients with first events per 100 PY. All data are for combined tofacitinib doses. *Excluding ORAL Surveillance. Data from ORAL Surveillance overall populations have previously been published and are included for reference; malignancies (excluding NMSC) and MACE (Ytterberg et al 1), MI (Charles-Schoeman et al 5). Also, previously published are data from the tofacitinib RA and PsA development programmes on MACE (Burmester et al 25) and VTE (Mease et al 26). IR, incidence rate; MACE, major adverse cardiovascular events; MI, myocardial infarction; N, number of evaluable patients; n, number of patients with events; NMSC, non-melanoma skin cancer; PsA, psoriatic arthritis; PY, patient-years; RA, rheumatoid arthritis; TNFi, tumour necrosis factor inhibitor; UC, ulcerative colitis; VTE, venous thromboembolism.