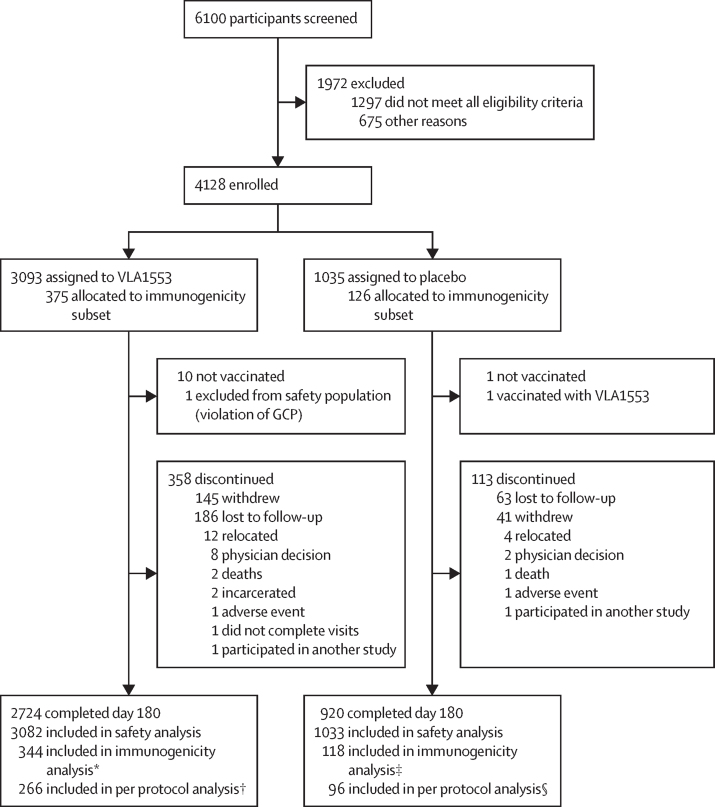
Figure 1.
Trial profile
The immunogenicity subset was recruited from 12 pre-selected study sites enrolling the first 501 participants entering the study. *31 of 375 participants were excluded from the immunogenicity subset for the following reasons: seropositive or unknown at baseline (n=17), no evaluable post-baseline titre (n=10), not vaccinated (n=4). †78 participants in the immunogenicity population were excluded from the per protocol subset for the following reasons: major protocol deviations (one or more of: out of visit windows [n=42], investigational product issue [n=20], missing endpoint data [n=15], exclusion criteria met [n=1], safety assessment issue [n=1], prohibited medication taken [n=1]). ‡Eight of 126 participants were excluded from the immunogenicity subset for the following reasons: no evaluable post-baseline titre (n=4), seropositive at baseline (n=4). §22 participants in the immunogenicity population were excluded from the per protocol subset for the following reasons: major protocol deviations (one or more of the following categories: out of visit windows [n=15], investigational product issue [n=4], missing endpoint data [n=3], prohibited medication taken [n=1]).