Abstract
Introduction
Progress towards leprosy elimination is threatened by increasing incidence in ‘hot-spot’ areas where more effective control strategies are urgently required. In these areas, active case finding and leprosy prevention limited to known contacts is insufficient for control. Population-wide active case-finding together with universal prevention through mass drug administration (MDA) has been shown to be effective in ‘hot-spot’ areas, but is logistically challenging and expensive. Combining leprosy screening and MDA with other population-wide screening activities such as for tuberculosis may increase programme efficiency. There has been limited evaluation of the feasibility and effectiveness of combined screening and MDA interventions. The COMBINE study aims to bridge this knowledge gap.
Methods and analysis
This implementation study will assess the feasibility and effectiveness of active leprosy case-finding and treatment, combined with MDA using either single-dose rifampicin or rifamycin-containing tuberculosis preventive or curative treatment, for reducing leprosy incidence in Kiribati. The leprosy programme will run over 2022–2025 in concert with population-wide tuberculosis screening-and-treatment in South Tarawa. The primary research question is to what extent the intervention reduces the annual leprosy new case detection rate (NCDR) in adults and children compared with routine screening and postexposure prophylaxis (PEP) among close contacts (baseline leprosy control activities). Comparisons will be made with (1) the preintervention NCDR separably among adults and children in South Tarawa (before–after study) and (2) the corresponding NCDRs in the rest of the country. Additionally, the postintervention prevalence of leprosy obtained from a survey of a ‘hot-spot’ sub-population will be compared with prevalence documented during the intervention. The intervention will be implemented in collaboration with the Kiribati National Leprosy Programme.
Ethics and dissemination
Approval has been obtained from the Kiribati Ministry of Health and Medical Services (MHMS), the University of Otago (H22/111) and the University of Sydney (2021/127) Human Research Ethics Committees. Findings will be shared with the MHMS, local communities and internationally through publication.
Keywords: leprosy, Hansen’s disease, mass drug administration, MDA, population, contacts, prevention, prophylaxis, single-dose rifampicin, SDR, intervention, post-exposure prophylaxis, PEP, active case finding, ACF, universal leprosy prophylaxis, mass chemoprophylaxis, rifapentine, Pacific, Kiribati
Strengths and limitations of this study.
Designed for both rapid and sustained reduction in leprosy prevalence using a combination of active case-finding with treatment and mass drug administration for population-wide chemoprophylaxis.
Geographically isolated island with high rates of leprosy, relatively small population and limited population mobility, facilitating proof-of-principle testing with low risk of dilution of intervention effect.
Dovetailing of existing leprosy and tuberculosis elimination activities has the potential to maximise efficiency and impact, especially in settings with a high-incidence of both diseases.
The absence of randomisation limits attribution of effect to the intervention; partially compensated for by employing multiple comparator assessments.
Despite the geographic isolation, the long implementation period (3 years) may allow leprosy reinfection events to occur in the community through inter-island travel.
Introduction
Since the 1991 World Health Association resolution to eliminate leprosy,1 tremendous progress has been made towards global leprosy elimination.2 However, despite enhanced early detection and availability of effective treatment and prevention options, progress has reversed in some leprosy ‘hot-spots’ (regions of high leprosy endemicity).3 National leprosy disease and disability rates have stagnated in most of the 23 leprosy global priority countries with an increase in grade 2 disability reported in 2020 for seven of these countries, including Kiribati.3 Global defunding for leprosy control and health system prioritisation of diseases with more obvious and immediate clinical presentations than leprosy have exacerbated these challenges. Point prevalence surveys in leprosy endemic regions reveal many undetected cases, with major case detection and reporting gaps responsible for the ‘missing millions’.4–10 Although the relatively low incidence of childhood leprosy (6.8% of all newly detected cases) indicates that transmission has declined globally, this is not true in all areas with cases among children increasing in some countries.3
The ongoing leprosy disease burden in the Pacific Island nation of Kiribati is emblematic of the global situation in high burden countries. Kiribati has one of the highest leprosy incidence rates in the world and these rates are on the rise; the Ministry of Health and Medical Services (MHMS) reports a 17% increase in incidence from 2010 to 2020, with 15.9 new cases detected per 10 000 people in 2020.3 Curative and preventive services are routinely provided by the National Leprosy Programme (NLP) in line with WHO guidelines, in partnership with the Pacific Leprosy Foundation (PLF). The NLP screens contacts for leprosy and, if active leprosy is not identified, provides single-dose rifampicin for post-exposure prophylaxis (SDR-PEP) immediately and 1 year later. In addition, contacts are screened for signs and symptoms of leprosy annually for four more years after the initial screening. SDR-PEP was introduced in 2018 and has since been provided to 89% of all eligible leprosy contacts recorded since 2010, which amounts to screening and prophylaxis for ~9% of the total population of Kiribati (10 406 contacts). Despite these interventions, most new leprosy cases in Kiribati are detected passively rather than by contact tracing with many presenting with advanced disease; almost half of all cases have multibacillary disease. These cases have long infectious periods before diagnosis and are an important source of transmission in the community.
To make an impact on the leprosy epidemic in Kiribati and to meet the ambitious Zero Leprosy target to halve global leprosy incidence by 2030,11 bold new strategies are needed. Such strategies should be designed to break the chain of leprosy transmission and to reduce the risk of disease progression in highly endemic regions. One avenue for exploration is to expand the reach of active case-finding (ACF) and preventive interventions in high-risk populations. In previous studies, regions with smaller populations, but similar disease burdens to Kiribati, have benefitted from population-wide ACF and mass drug administration (MDA) with SDR to reduce the risk of progression to leprosy disease in the community, irrespective of contact status.12 13 Population-wide programmes can be very challenging to implement on a large scale because of the logistical demands of reaching whole populations, difficulties achieving acceptability and buy-in, poor access to microbial confirmation in resourced-limited settings, a lack of clinical expertise for diagnoses and challenges in mobilising resources to support population-wide programmes. The result is that leprosy MDA for large populations (>5000 people) is often considered unfeasible in the regions where it is most needed.
Twenty-one of the 23 leprosy priority countries also have endemic tuberculosis (TB).3 The relatively greater funding for TB and the global movement towards expanded ACF for TB,14–16 the shared susceptibility of Mycobacterium leprae and Mycobacterium tuberculosis to rifamycins for preventive therapy, and the similar social determinants of transmission and disease all present opportunities for leprosy control programmes to leverage TB programmes for mutual gain. Where the burden of both diseases is sufficiently high, this can take the form of combined population-wide ACF and MDA chemoprophylaxis activities. In South Tarawa, the PEARL study (Pathway to the Elimination of Antibiotic Resistant and Latent tuberculosis (as well as leprosy) in the Pacific)17 provides the mechanism by which a combined intervention may be delivered at a fraction of the cost of a separate programme. Modelling of a mass chemoprophylaxis strategy for leprosy suggests this is an effective strategy,18 and combining mass screening and treatment for TB are expected to greatly increase efficiency and cost-effectiveness. South Tarawa was chosen for the PEARL study as it is the centre with the highest population density in Kiribati and has the highest estimated incidence of TB and leprosy.
The COMBINE study is designed to inform programmatic strategies towards leprosy elimination in the Pacific and elsewhere. We aim to assess the effectiveness, feasibility, efficiency and cost of a programme of leprosy screening and mass rifamycin-based chemoprophylaxis delivered in combination with a TB screening, treatment and prevention initiative in Kiribati.17 We will provide evidence for practicable means of integrating leprosy control with other communicable disease programmes that can be used to effectively accelerate leprosy prevention and care in endemic regions. Many of the research questions addressed by the COMBINE study must be answered to achieve scalable and durable leprosy elimination in countries like Kiribati.
Objectives
The COMBINE study assesses the feasibility and effectiveness of leprosy screening and MDA chemoprophylaxis in a highly endemic population using a programmatic approach that:
Investigates whether combined population-wide screening and treatment for leprosy and TB together with MDA chemoprophylaxis and ongoing SDR-PEP for contacts can achieve rapid and durable reductions in leprosy incidence.
Evaluates the effectiveness of leprosy MDA chemoprophylaxis using a pragmatic combination of either SDR or rifamycin-based TB preventive treatment.
Measures the cost of MDA delivery when integrated with infrastructure from an existing population-wide screening programme (the PEARL study17).
Documents operational strategies to feasibly integrate enhanced leprosy and TB control efforts, and to reduce leprosy-associated stigma.
Methods and analysis
Study design
COMBINE is a pragmatic controlled non-randomised before-and-after implementation study designed to evaluate the impact of the intervention on leprosy new case detection rate (NCDR). The COMBINE study will leverage infrastructure created by the PEARL study to deliver population-wide leprosy ACF and chemoprophylaxis. We will deliver the intervention over 3 years commencing November 2022 and ending November 2025, aiming to reach the entire population of South Tarawa in that time. The timeline of planned activities for the COMBINE study is illustrated in online supplemental figure 1.
bmjopen-2022-065369supp001.pdf (386KB, pdf)
Setting
The Republic of Kiribati is a geographically isolated nation in the Pacific region comprising 32 atolls and one raised coral island spread over a land territory of 811 km2 amid an ocean territory of 3.5 million km2. The intervention site is the capital atoll of South Tarawa (population 63 439) which is the densely populated ‘transmission hot-spot’ and amplifier of leprosy disease throughout the country (see table 1 for baseline characteristics of the study population).19 Kiribati has only one specialised leprosy clinic which is located in South Tarawa. Residents live in village communities on a chain of low-lying islets connected by a causeway. Visitors from ‘outer islands’ to the capital often stay for an extended period. Anecdotally, this pattern of travel in and out of South Tarawa is associated with clusters of TB and leprosy in outer island communities.
Table 1.
Baseline characteristics of the population and intervention group
| Intervention group South Tarawa |
No intervention group Rest of Kiribati |
Whole of Kiribati | |
| Population, 2020* | 63 439 | 56 501 | 119 940 |
| Females (%) | 32 981 (52.0%) | 27 805 (49.2%) | 60 786 (50.7%) |
| Median age (years) | 23.2 | 22.3 | 22.9 |
| Average household size (people) | 6.6 | 4.9 | 5.0 |
| Net migration rate (% of population) |
2% | 0.7% | 1.4% |
| Urbanisation | Majority urban; some rural | Majority rural; some urban | Mixed urban and rural |
| BCG coverage, 2021 (% of live births) † |
2434/2525 (96.4%) | 839/888 (94.5%) | 3273/3413 (95.9%) |
| Leprosy new cases, 2020 (rate per 10 000) † |
93 (14.44) | 62 (11.15) | 155 (12.92) |
| Child cases (%) | 18 (19.4%) | 18 (29.0%) | 36 (23.2%) |
| MB cases (%) | 50 (53.8%) | 20 (32.3%) | 70 (45.2%) |
| Eligible contacts 2010–2020 † | 9527 | 2264 | 11 811 |
| Received SDR (%) | 8381 (88%) | 2021 (89%) | 10 402 (89%) |
Selected baseline characteristics or populations in the intervention area (South Tarawa), no intervention area (rest of Kiribati) and for the whole of Kiribati.
*National Statistics Office, 2020 census data.
†Ministry of Health and Medical Services, programme data.
BCG, Bacille Calmette-Guerin; MB, multibacillary; SDR, single-dose rifampicin.
While diagnosis and contact-tracing practices have been improved and standardised since 2010, it is uncertain whether the upward trend in NCDR in Kiribati over the past decade is an accurate measure of worsening epidemic control or reflective of enhanced case detection. What is clear is that child NCDR has exceeded 30% of all newly detected cases for the past 3 years (2019–2021), indicating that the background community-level leprosy transmission has outpaced the potential to control the disease burden with existing leprosy programme interventions. Our study reports NCDR among both adults and children to examine variations in leprosy distribution between these two groups.
Intervention group and recruitment
The intervention group comprises residents of South Tarawa (and the small communities of Buota and Abatao adjacent to South Tarawa) aged 3 years and above, and aged less than 3 years if they have documented household contact (relevant definitions are provided in box 1) with someone who has had TB in the past 1 year, or leprosy at any time since they were born. Study participants will be identified via household and village-level lists of residents from the 2020 census, and then invited to attend screening locations using door-to-door visits at households and community-based institutions (businesses, churches, etc). Basic demographic, social and geographic data will be collected at enrolment by the PEARL study screening teams.
Box 1. Definitions.
Case of leprosy: clinical definition classified as multibacillary (MB) or paucibacillary (PB) according to WHO criteria that has been diagnosed by the doctor of the National Leprosy Programme (NLP).
Household: all those using the same kitchen, including members of extended families, the maneaba (communal hospitality shelters) and dormitories in individual locations.
Household contact: any person who has been in contact with a new leprosy case for at least 20 hours per week for at least 3 months during the past 5 years.*
*Adapted from WHO definition for the Kiribati context.
Interventions
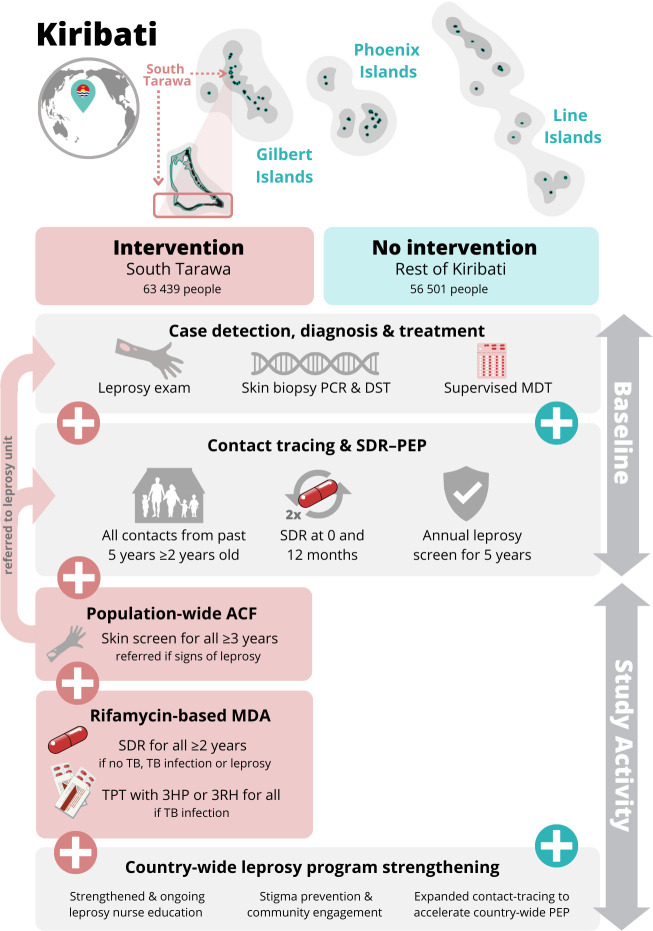
An illustration of the combined TB and leprosy interventions is provided in figure 1, comparing the intervention group and standard care in the comparison group. Interventions are described in detail below. In practice, these interventions will be delivered in the setting of a combined community-based screening, diagnosis, treatment and prevention service.
Figure 1.
Overview of COMBINE study intervention. Overview of COMBINE study activities, comparing interventions delivered in South Tarawa (pink, intervention group) and in the rest of Kiribati (green, no intervention group). Activities are further divided according to those available at baseline across the country and continued during the study period, and those activities that will be delivered during the study period (vertical arrows at right). Geographical context is illustrated at top (not to scale). 3HP, 3 months of weekly isoniazid and rifapentine; 3RH, 3 months of daily isoniazid and rifampicin; ACF, active case finding; DST, drug susceptibility testing; MDA, mass drug administration; MDT, multidrug treatment; NLP, National Leprosy Programme; PEP, postexposure prophylaxis; SDR, single-dose rifampicin; TB, tuberculosis; TPT, TB preventive treatment.
Case detection, diagnosis and treatment
Screening for leprosy will be conducted by a physical examination and questionnaire (online supplemental material 1). People with presumptive leprosy will be referred to the NLP for expert diagnosis. Cases will be validated by a leprologist and skin biopsies from all patients with clinically diagnosed leprosy will be tested by PCR for M. leprae and drug resistance mutations, according to WHO guidelines.14 Leprosy treatment will be provided by the NLP according to Kiribati national guidelines. Further details of the leprosy screening, diagnosis and treatment eligibility criteria are available in the PEARL study protocol.17
Contact tracing and PEP
Contact tracing and SDR-PEP will be ongoing for all index cases identified during the intervention within the study site and throughout the rest of Kiribati, as is consistent with routine practice (box 1). WHO recommends that leprosy contacts should be given SDR-PEP at ≥2 years of age.20 This has been adopted by the Kiribati NLP since 2018 and will be supported by the COMBINE study to scale-up SDR-PEP delivery throughout the intervention period, as enhanced index case detection will increase contact tracing needs. Children who are younger than 2 years and are leprosy contacts will be followed up and offered SDR-PEP by the NLP when they reach 2 years of age.
Leprosy MDA chemoprophylaxis
Rifamycin-based MDA chemoprophylaxis is provided using a composite of treatments. After integrated leprosy and TB screening, participants will be commenced on treatment for TB, treatment for leprosy or TPT using a rifamycin-based regimen, depending on the screening outcome. For participants who are not eligible for any of those treatments, we will then offer a single dose of rifampicin according to the inclusion/exclusion criteria in table 2. Considered together as in table 3, all participants will be offered a rifamycin-based treatment; effectively a rifamycin-based leprosy MDA chemoprophylaxis. Detailed dosing information is provided in online supplemental material 2. SDR for PEP and MDA will be provided without baseline blood tests, consistent with the standard of care in Kiribati.
Table 2.
Inclusion and exclusion criteria for single dose rifampicin
| Inclusion criteria | Exclusion criteria |
| 1. Enrolled in the screening intervention | 1. History of serious liver or kidney disease. |
| 2. Not eligible for any other rifamycin-based treatment | 2. Known pregnancy (SDR can be given after delivery). |
| 3. Aged ≥2 years | 3. Known allergy or severe adverse effects experienced with rifampicin use. |
| 4. Informed consent. For children (<18 years) consent will be obtained from the parent or guardian, and children (≥10 years) will also provide assent. | 4. Refuses participation. |
Inclusion criteria are shown for single dose rifampicin. Other treatment regimens are determined by indications and contraindications relevant to those regimens.
SDR, single-dose rifampicin.
Table 3.
Overview of combined treatment and chemoprophylaxis
| Screening outcome | Treatment offered | Rifamycin component |
| Leprosy | Leprosy MDT | Monthly rifampicin for 6–12 months |
| TB | TB treatment | Daily rifampicin for 6–12 months |
| RR-TB | DR-TB treatment+SDR | Single dose rifampicin |
| Eligible for TPT | 3HP or 3RH | Weekly rifapentine or daily rifampicin for 3 months |
| Leprosy HHC | SDR-PEP | Rifampicin given at baseline and 1 year later |
| None of the above | SDR-MDA | Single dose of rifampicin |
Screening outcomes are exhaustive but not mutually exclusive. The rifamycin component of each treatment strategy includes sufficient exposure to offer prevention for leprosy, in effect a MDA of leprosy chemoprophylaxis.
DR-TB, drug-resistant tuberculosis; HHC, household contact; 3HP, 3-months of weekly rifapentine and isoniazid; MDA, mass drug administration; MDT, multidrug treatment; NLP, national leprosy program; PCR, polymerase chain reaction; PEP, postexposure prophylaxis; 3RH, 3-months of daily rifampicin and isoniazid; RR-TB, rifampicin-resistant tuberculosis; SDR, single-dose rifampicin; TB, tuberculosis; TPT, tuberculosis preventive treatment.
Community engagement and stigma prevention
The objective of community engagement and mass communications is to encourage participation in the screening programme and sensitise community members to appropriate, non-stigmatising messages related to leprosy and leprosy screening. This approach is supported by the best practice statement of the Global Partnership for Zero Leprosy.11 21 The COMBINE study supports community engagement and stigma prevention through various activities developed in concert with the PLF (which have 10 years of experience in leprosy advocacy in Kiribati) and the MHMS. These activities include:
Biannual advocacy activity drives which may include leprosy awareness parades, plays, signage and mass communication.
Convening of a leprosy community support group for patients diagnosed with leprosy and their close contacts/families. COMBINE nurses will assist with mentoring the community group, training in coping strategies and supporting activities.
Annual training and development workshops including all staff of the national leprosy and TB programmes with antistigma training for health staff delivering the COMBINE screening intervention.
Job aids and resources to support health staff and people with leprosy, for example a flipbook to aid counselling sessions between health workers and people with leprosy.
Post-intervention prevalence survey
A follow-up leprosy prevalence survey will be conducted in Betio islet (~18 500 people, located within the South Tarawa intervention group), 3–4 years after the intervention has been completed.
Outcome measures and planned analyses
The primary research question of interest is the extent to which the intervention reduces leprosy annual adult and child NCDR compared with standard routine passive case-finding and PEP of close contacts. This will be assessed (1) by comparing the postintervention NCDRs in South Tarawa (in 2025) with the preintervention NCDRs (in 2021) and (2) by comparing the change in adult and child NCDRs in South Tarawa (the intervention site) with the change in NCDRs observed in the outer Kiribati islands (non-intervention sites). A supplementary analysis will compare the prevalence rate of leprosy in Betio (~15 000 people) found in the initial population-wide screening intervention with the rate found from a survey in the same population performed 3–4 years later. All primary, supplementary and planned analyses will be performed using standard statistical methods, for example using Poisson regression for the NCDR outcomes and logistic regression to compare prevalence during and after the intervention.
Due to the long latency of leprosy, we expect that the full effect of the intervention will only become apparent after several years have elapsed. MHMS and PLF are committed to continuing leprosy surveillance in Kiribati, enabling ongoing assessments of long-term trends in disease burden beyond 2025.
Other planned analyses will examine:
Diagnostic yield of leprosy screening using an optimised clinical examination and brief history in the setting of a community-based multidisease screening intervention. Examinations of yield among discrete age bands in children (0–4, 5–9, 10–14 years) will also indicate effect of the intervention on transmission over time.
Description of the spectrum of leprosy disease in South Tarawa.
Description of leprosy clusters and transmission patterns using geospatial data and social contact mapping.
The prevalence ratio of genotypic M. leprae resistance to rifampicin, dapsone and quinolones before and after the MDA programme (using PCR-based assays of skin biopsies).22–25
The costs of leprosy-only activities, TB-only activities and shared activities to inform the cost-efficiency of future combined leprosy elimination projects in the Pacific and elsewhere.
The relative risk of leprosy diagnosis in participants with and without prior exposure to rifamycins (provided as part of routine activities of the NLP and NTP), prior BCG vaccination and prior TB infection and/or disease in a high-incidence setting.
The feasibility of combined TB and leprosy elimination efforts in the intervention site assessed using a mixed-methods approach: measurement of treatment coverage, description of health service requirements (healthcare worker mix and person-time), description of infrastructure requirements and surveys and interviews conducted with healthcare workers, decision-makers, community representatives and study participants.
Mathematical modelling of the dynamics of leprosy incidence using ‘real life’ data from the COMBINE study with the aim of refining previous models18 to improve accuracy of forecasting and decision support.
Sample size
Assuming mass chemoprophylaxis coverage of 80% of the population and given an overall leprosy NCDR of 1600/1 000 000 for South Tarawa (the intervention group, population ~65 000) and 872 per 1 000 000 for the rest of the country (comparison group, population ~55 000), the study would provide greater than 99% power (α≤0.05) to detect a 50% reduction (before vs after difference) in NCDR in South Tarawa (the intervention group) and 82% power to detect a 50% reduction in South Tarawa compared with no change in the rest of the country. Predicted sample sizes were calculated using the mean number of cases observed between 2018 and 2020 and simulated number of cases observed in 2025, drawn from a Poisson distribution (10 000 replicates) according to the parameters above and assuming a population growth of 5000 in each area.
Economic analysis and costing
COMBINE proposes to estimate unit costs for screening per patient, working closely with the PEARL study to perform a cost analysis of TB-only activities, leprosy-only activities and shared activities. Accurate costing data will inform future leprosy elimination projects in the Pacific region and beyond, and will have additional benefits for subsequent planning, budgeting and modelling exercises. To enhance the application of our findings, we will seek to align costing data with existing interagency costing tools.
Data collection and monitoring
All leprosy and TB screening, chemoprophylaxis and outcome data will be captured offline on encrypted tablet devices using Research Electronic Data Capture (REDCap) surveys. Data will be uploaded and stored on a high security REDCap database server managed by the University of Sydney. Leprosy case and contact management data are already archived in a comprehensive NLP database, with maintenance supported by the PLF before, during and after the study. We will contribute case and contact data from the COMBINE study to the existing supported database through routine study procedures. Mass screening and MDA data will be available to NLP as needed and handed over to the NLP after study completion.
Poststudy follow-up activities
Country-wide ACF and PEP for household contacts will continue beyond the COMBINE study as a joint programme implemented by the NLP and the PLF. We consider that the early findings of the present study will enable mobilisation of funds to deliver similar population-wide leprosy control activities in other parts of the country, as part of a ‘Zero Leprosy Roadmap’. Case and contact management records are already maintained in a comprehensive database, and relevant data from the COMBINE study will be added as part of study procedures. Together with mass screening data, this will provide a rich source for future analysis of long-term outcomes in the study population.
Ethics and dissemination
Ethical approval for the COMBINE study has been obtained from the University of Sydney (project no. 2021/127), the University of Otago (H22/111) and the Kiribati Ministry of Health and Medical Services. Government support for the study to occur in collaboration with the NLP has been provided by Kiribati National Cabinet.
Participant information and counselling is provided to all prospective participants and again before treatment is offered (online supplemental material 3). Editable versions of study patient tools and a counselling flipbook are available at www.leprosy.org.nz and www.thepearlstudy.org. Informed consent is gathered verbally before participant enrolment and a signed record of consent is collected in the study REDCap database (online supplemental material 4). Verbal consent for participants attending the NLP for confirmation of diagnosis and treatment management will be obtained according to standard programme practice. Written informed consent for a skin biopsy will be taken as is usual in the clinic. This includes consent to send the specimen abroad for analysis.
The safety of treatments for TB are discussed in the PEARL study protocol.17 SDR is very safe, and has been used in Kiribati18 19 and elsewhere12 26–28 with little or no recorded side-effects. A study hotline and walk-in clinic will be freely available for adverse event (AE) management throughout the study period. Information on the signs and symptoms of leprosy and instructions to access the permanent leprosy clinic are provided to all participants (including those who decline to participate). Serious AEs are reported in accordance with national and University of Sydney pharmacovigilance standards. Intervention monitoring and auditing procedures will be conducted annually by the MHMS in accordance with routine practice with study reports made annually to ethics and funding bodies.
All study data will be shared with the MHMS. Reports of study progress will be made to the Kiribati community by mass communication and on the PLF and the PEARL study websites. Study findings will be presented at international conferences and in peer-reviewed publications.
Patient involvement statement
This study was developed with the involvement of a reference group of I-Kiribati people affected by leprosy. This group will be involved in the COMBINE study in an ongoing basis. In particular, they will give advice concerning the practical implementation of the research, including the best ways of liaising with patients, their families and the community to ensure that communication is positive and does not contribute to stigma for people identified with leprosy and their contacts.
Discussion
Innovative solutions are required to achieve progress towards leprosy elimination in Kiribati and in other areas of continuing high incidence. This is essential if we are to overcome the barriers to achieving global Zero Leprosy targets,11 and eventually, true global leprosy elimination. As leprosy continues to require long and complex treatment programmes, there is also urgency to find novel control strategies before antibiotic resistance emerges and accelerates in high transmission populations.29
The proposed study design combines a robust public health intervention in response to the dual epidemics of leprosy and TB in Kiribati, along with rigorous evaluation of the intervention. Population-wide leprosy ACF and MDA with rifamycin-based chemoprophylaxis in a population of approximately 60 000 represents a bold step towards acceleration of leprosy control. Combining this intervention with a similar population-wide TB screening, treatment and prevention programme is an innovative health systems approach that could improve efficiency and feasibility of large-scale interventions for both diseases. If successful, this would present an important model that may be implemented in other settings.
There are several limitations associated with this study protocol that may affect outcomes. First, the population wide screening and MDA intervention in this study is in essence a change in health policy whereby the target population is expanded to include all residents of South Tarawa, rather than just specific contacts or groups of individuals. As with any health policy change or community-facing intervention, the impact of this new approach is dependent on the new policy reaching a large proportion of the target population and being delivered with high fidelity to the proposed design. We will take every effort to achieve high uptake and retention in care by conducting extensive community mobilisation and health communication activities. Although the outcomes of this study are defined at population level (NCDR measured before and after) and not dependent on individual level enrolment and withdrawal, we will also maintain detailed individual level records to enhance follow-up. Second, the intervention is not randomised. This will limit our ability to make inferences about causation, especially if the impact on NCDR is small. We hope that by including the rest of the country as a non-randomised ‘control’ group we will have some basis for comparison. Finally, the proposed combined approach to screening and prevention means that the intervention is more time consuming to deliver and will take longer to reach the entire target population. We anticipate some level of reinfection to occur in already-screened areas of South Tarawa, while we continue to deliver the intervention across the island. Although reinfections may reduce the impact on measured NCDR, we anticipate that this would still be valuable information since we are taking a ‘real world’ public health approach that could serve as an example to other settings.
In Kiribati (and elsewhere12 30), MDA chemoprophylaxis in the 1990s led to reductions in leprosy case detection but ultimately failed to produce a lasting decline in incidence in some settings. The long latency period of the disease and the absence of surrogate measures of leprosy transmission make robust short-to-medium-term outcome measures of the population-level effect of interventions particularly limited. These are challenges intrinsic to population-level leprosy research. This study is designed to address these challenges by rigorous evaluation of scaled-up interventions in combination with durable partnership for evaluation of longer-term incidence and transmission outcomes. This ‘real-life’ operational research design to evaluate the main intervention is accompanied by a commitment by the MHMS together with the PLF to deliver leprosy control activities over the longer term, until leprosy is eliminated in Kiribati (www.leprosy.org.nz); this includes surveillance, continuation of rigorous contact identification and management and expansion of population-wide screening and MDA to the rest of the country. In the shorter and medium term, improvements in the accuracy of modelling of the intervention impact (using data from the COMBINE study) will provide useful insights and interim measures of the effect of COMBINE interventions on leprosy incidence. This will be valuable to inform programmes facing similar challenges to Kiribati. The present study, along with short, medium and long-term aims and commitments are currently being integrated using a ‘Leprosy elimination roadmap’, adapting the methods and experiences of the Global Partnership for Zero Leprosy. By embedding this study within long-term strategic partnerships, with ongoing funding and a comprehensive strategy, we hope that the mis-steps of previous MDA interventions can be avoided and lasting impact can be achieved along with research outputs that will guide future interventions.19
Kiribati is in a unique position, given its geographic isolation, low migration rate and limited population size, to identify and test innovative elimination strategies as proof-of-principle for leprosy control in other locations. We plan to grasp this opportunity and deliver much-needed evidence to reinvigorate attempts to eliminate this age-old scourge of humankind.
Supplementary Material
Acknowledgments
We thank the chair of the Leprosy Reference Group in Kiribati, Ms Nanoo Maribo, for her support of the COMBINE study and the Kiribati National Leprosy Program for their outstanding contributions to this work. We thank Ms Kantaake Corbett, Kiribati Health Information Unit, Ms Aritita Tekaieti, Kiribati National Statistics Office, and Mr Luis de la Rua, Pacific Community Statistics for Development Division, for their assistance with baseline data
Footnotes
Twitter: @_MikaelaColeman
Contributors: All authors contributed to the conceptualisation and panning of this research. MC, JH, NMD, JT, JW, BJM, WJB and STC wrote the study proposal. ET, ER, TB, NI, AC, NMD, TI, POC and PP made important intellectual contributions to the final protocol manuscript. MC and JH contributed equally to this paper. The funding agency played no part in any aspect of the study, nor the decision to submit this manuscript for publication.
Funding: The COMBINE study is funded by the Leprosy Research Initiative with 50% co-funding from the Turing foundation. Additional funding to support the collaborating National Leprosy Program is provided by the Pacific Leprosy Foundation, New Zealand. This work is a partnership with the PEARL study which is funded by the Australian Medical Research Future Fund (APP1200755)
Competing interests: Some project funding is provided by the Pacific Leprosy Foundation, which also supports consultancy work of coauthors AC and NI.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.World Health Assembly . Resolution to eliminate leprosy as a public health problem by the year 2000 resolution WHA449. Geneva: 44th World Health Assembly, 1991. [Google Scholar]
- 2.WHO . World Health Organization leprosy elimination project: status report 2002: world health organization; 2003.
- 3.WHO . World health organization global leprosy (Hansen disease) update, 2020: impact of COVID-19 on global leprosy control. Weekly Epidemiol Rec 2021;96:421–44. [Google Scholar]
- 4.Urgesa K, Bobosha K, Seyoum B, et al. Evidence for hidden leprosy in a high leprosy-endemic setting, Eastern Ethiopia: the application of active case-finding and contact screening. PLoS Negl Trop Dis 2021;15:e0009640. 10.1371/journal.pntd.0009640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernardes F, Paula N de, Leite MN, et al. Evidence of hidden leprosy in a supposedly low endemic area of Brazil. Mem Inst Oswaldo Cruz 2017;112:822–8. 10.1590/0074-02760170173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith WC, van Brakel W, Gillis T, et al. The missing millions: a threat to the elimination of leprosy. PLoS Negl Trop Dis 2015;9:e0003658. 10.1371/journal.pntd.0003658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Oliveira GL, Oliveira JF, Pescarini JM, et al. Estimating underreporting of leprosy in Brazil using a Bayesian approach. PLoS Negl Trop Dis 2021;15:e0009700. 10.1371/journal.pntd.0009700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frade MAC, de Paula NA, Gomes CM, et al. Unexpectedly high leprosy seroprevalence detected using a random surveillance strategy in midwestern Brazil: a comparison of ELISA and a rapid diagnostic test. PLoS Negl Trop Dis 2017;11:e0005375. 10.1371/journal.pntd.0005375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mangeard-Lourme J, Singh A, Singh RK, et al. Enhanced active case-finding, identifying leprosy cases missed by recent detection campaigns in Munger district, Bihar, India. Lepr Rev 2017;88:452–62. 10.47276/lr.88.4.452 [DOI] [Google Scholar]
- 10.Salgado CG, Barreto JG, da Silva MB, et al. Are leprosy case numbers reliable? Lancet Infect Dis 2018;18:135–7. 10.1016/S1473-3099(18)30012-4 [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization . TowardsZero leprosy. global leprosy (Hansen’s disease) strategy 2021–2030; 2021.
- 12.Tiwari A, Dandel S, Djupuri R, et al. Population-wide administration of single dose rifampicin for leprosy prevention in isolated communities: a three year follow-up feasibility study in Indonesia. BMC Infect Dis 2018;18:324. 10.1186/s12879-018-3233-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bakker MI, Hatta M, Kwenang A, et al. Prevention of leprosy using rifampicin as chemoprophylaxis. Am J Trop Med Hyg 2005;72:443–8. [PubMed] [Google Scholar]
- 14.WHO . World health organization consolidated guidelines on tuberculosis Module 2: screening – systematic screening for tuberculosis diseas. Global Tuberculosis Programme; 2021. [PubMed] [Google Scholar]
- 15.WHO . Rapid communication on systematic screening for tuberculosis. 2020. [Google Scholar]
- 16.Marks GB, Nguyen NV, Nguyen PTB, et al. Community-wide screening for tuberculosis in a high-prevalence setting. N Engl J Med 2019;381:1347–57. 10.1056/NEJMoa1902129 [DOI] [PubMed] [Google Scholar]
- 17.Coleman M, Hill J, Timeon E, et al. Population-wide active case finding and prevention for tuberculosis and leprosy elimination in Kiribati: the PEARL study protocol. BMJ Open 2022;12:e055295. 10.1136/bmjopen-2021-055295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilkison C, Chambers S, Blok DJ, et al. Predicting the impact of household contact and mass chemoprophylaxis on future new leprosy cases in South Tarawa, Kiribati: a modelling study. PLoS Negl Trop Dis 2019;13:e0007646. 10.1371/journal.pntd.0007646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chambers ST, Ioteba N, Timeon E, et al. Surveillance of leprosy in Kiribati, 1935–2017. Emerg Infect Dis 2020;26:833–40. 10.3201/eid2605.181746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WHO . Leprosy/Hansen disease: contact tracing and post-exposure prophylaxis. 2020. [Google Scholar]
- 21.van Brakel WH, da Silva Pereira ZB, Barbosa JC, et al. Global partnership for zero leprosy research agenda working group subgroup on stigma; 2019.
- 22.Coppola M, van den Eeden SJF, Robbins N, et al. Vaccines for leprosy and tuberculosis: opportunities for shared research, development, and application. Front Immunol 2018;9:308. 10.3389/fimmu.2018.00308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lahiri R, Adams LB. Cultivation and viability determination of Mycobacterium leprae. International Textbook of Leprosy, 2016. [Google Scholar]
- 24.Davis GL, Ray NA, Lahiri R, et al. Molecular assays for determining mycobacterium leprae viability in tissues of experimentally infected mice. PLoS Negl Trop Dis 2013;7:e2404. 10.1371/journal.pntd.0002404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams DL, Torrero M, Wheeler PR, et al. Biological implications of mycobacterium leprae gene expression during infection. J Mol Microbiol Biotechnol 2004;8:58–72. 10.1159/000082081 [DOI] [PubMed] [Google Scholar]
- 26.Richardus JH, Tiwari A, Barth-Jaeggi T, et al. Leprosy post-exposure prophylaxis with single-dose rifampicin (LPEP): an international feasibility programme. Lancet Glob Health 2021;9:e81–90. 10.1016/S2214-109X(20)30396-X [DOI] [PubMed] [Google Scholar]
- 27.Schoenmakers A, Mieras L, Budiawan T, et al. The state of affairs in post-exposure leprosy prevention: a descriptive meta-analysis on immuno- and chemo-prophylaxis. Res Rep Trop Med 2020;11:97–117. 10.2147/RRTM.S190300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feenstra SG, Pahan D, Moet FJ, et al. Patient-related factors predicting the effectiveness of rifampicin chemoprophylaxis in contacts: 6 year follow up of the COLEP cohort in Bangladesh. Lepr Rev 2012;83:292–304. 10.47276/lr.83.3.292 [DOI] [PubMed] [Google Scholar]
- 29.Cambau E, Saunderson P, Matsuoka M, et al. Antimicrobial resistance in leprosy: results of the first prospective open survey conducted by a WHO surveillance network for the period 2009–15. Clin Microbiol Infect 2018;24:1305–10. 10.1016/j.cmi.2018.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen LN, Cartel JL, Grosset JH. Chemoprophylaxis of leprosy in the Southern Marquesas with a single 25 mg/kg dose of rifampicin. results after 10 years. Lepr Rev 2000;71 Suppl:S33–5. 10.5935/0305-7518.20000064 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-065369supp001.pdf (386KB, pdf)