Abstract
Background:
Literature suggests that maternal exposure to persistent organic pollutants (POPs) may influence child neurodevelopment. Evidence linking prenatal POPs and autism spectrum disorder has been inconclusive and few studies have examined the mixture effect of the POPs on autism-related traits.
Objective:
To evaluate the associations between prenatal exposure to a mixture of POPs and autism-related traits in children from the Early Autism Risk Longitudinal Investigation study.
Methods:
Maternal serum concentrations of 17 POPs (11 polychlorinated biphenyls [PCBs], 4 polybrominated diphenyls [PBDEs], and 2 persistent pesticides) in 154 samples collected during pregnancy were included in this analysis. We examined the independent associations of the natural log-transformed POPs with social, cognitive, and behavioral traits at 36 months of age, including Social Responsiveness Scale (SRS), Mullen Scales of Early Learning-Early Learning Composite (MSEL-ELC), and Vineland Adaptive Behavior Scales (VABS) scores, using linear regression models. We applied Bayesian kernel machine regression and quantile g-computation to examine the joint effect and interactions of the POPs.
Results:
Higher ln-PBDE47 was associated with greater deficits in social reciprocity (higher SRS score) (β=6.39, 95% CI: 1.12, 11.65) whereas higher ln-p,p’-DDE was associated with lower social deficits (β=−8.34, 95% CI: −15.32, −1.37). Positive associations were observed between PCB180 and PCB187 and cognitive (MSEL-ELC) scores (β=5.68, 95% CI: 0.18, 11.17; β=4.65, 95% CI: 0.14, 9.17, respectively). Adaptive functioning (VABS) scores were positively associated with PCB170, PCB180, PCB187, PCB196/203, and p,p’-DDE. In the mixture analyses, we did not observe an overall mixture effect of POPs on the quantitative traits. Potential interactions between PBDE99 and other PBDEs were identified in association with MSEL-ELC scores.
Conclusions:
We observed independent effects of PCB180, PCB187, PBDE47, and p,p’ DDE with ASD-related quantitative traits and potential interactions between PBDEs. Our findings highlight the importance of assessing the effect of POPs as a mixture.
Keywords: persistent organic pollutants, autism-related traits, environmental mixture, Bayesian kernel machine regression, quantile g-computation
1. Introduction
Autism Spectrum Disorder (ASD) is a neurodevelopmental condition and a major public health burden affecting 1 in 44 children in the US (Maenner et al. 2021). Currently the etiology of ASD is not fully understood. Large-scale genomic studies have identified a number of rare inherited and de novo mutations and common genetic variants associated with ASD (Gaugler et al. 2014; Grove et al. 2019; Iossifov et al. 2014). Recent studies also show that the perinatal period represents a critical developmental window and prenatal exposure to environmental factors, particularly environmental toxicants, are linked to increased risk of ASD (Hertz-Picciotto et al. 2018; Rossignol et al. 2014; Ye et al. 2017).
Persistent organic pollutants (POPs) are a class of compounds that settle in the environment for an extensive amount of time and can bio-accumulate in the food chain. Examples of these compounds include polychlorinated biphenyls (PCBs), polybrominated diphenyls (PBDEs) and perfluorooctanoic acid (PFOA). Although many POPs have been banned in the US, levels of these chemicals can still be detected in human serum samples (Chen et al. 2017; Sjodin et al. 2014; Thompson and Boekelheide 2013). Many POPs have established adverse effects on neurodevelopment. Several potential pathways have been hypothesized to underlie the mechanisms through which POPs may influence child neurodevelopment including endocrine or immune system disruption, thyroid hormone levels, and epigenetic effects (Ashwood et al. 2009; de Escobar et al. 2004; Dunaway et al. 2016; Hertz-Picciotto et al. 2008; Laufer et al. 2022; Liu et al. 2012). Previous studies have investigated prenatal exposure to POPs as a potential risk factor for ASD and social, cognitive, and behavioral outcomes and observed associations of specific POP congeners and/or groups of POP classes with several ASD-related outcome (Braun et al. 2014; Cheslack-Postava et al. 2013; Granillo et al. 2019; Lyall et al. 2017a; Lyall et al. 2017b). However, the findings of those studies are not consistent. More recently, with the development of advanced statistical methods, researchers have moved toward evaluating the joint effects of multiple chemicals as co-exposures and interactions among the chemicals. Prior work has examined the mixture effect of POPs on ASD or child social and behavioral development outcomes (Braun et al. 2014; Hamra et al. 2019). One study did not observe a relationship between ASD and POP congeners together with other chemicals in a mixture analysis using a Bayesian approach (Hamra et al. 2019). A recent publication proposed a flexible Bayesian approach as Bayesian Weighted Sums (BWS) and applied it to simulated data and the Early Autism Risk Longitudinal Investigation (EARLI) study. The authors reported positive associations between summed PBDEs and social deficits and ASD risk (Hamra et al. 2021). Given that environmental chemicals can co-occur in time or space, it is crucial to incorporate novel mixture models to account for the complex correlations and interactions among the individual environmental chemicals.
In this study, we examined the association between prenatal exposure to a mixture of POPs and quantitative neurodevelopmental traits including related social communication, and cognitive and adaptive function at 36 months of age in the EARLI study. These outcome measures have relevance across neurodevelopmental diagnoses and may provide insight into how population shifts in distributions impact health. Building upon previous evidence and findings in the EARLI study, we applied linear regression to investigate the independent effects and Bayesian kernel machine regression (BKMR) and quantile g-computation to investigate the individual effects and the joint effects of the POP mixtures and potential interactions between the POPs. These analyses will allow us to understand how individual POPs and groups of POPs impact health.
2. Methods
2.1. Study Population
Participants for this analysis were enrolled in the EARLI study. Details of the EARLI study have been previously described (Newschaffer et al. 2012). Briefly, the EARLI study is a prospective study of ASD using a high familial likelihood (HFL) design. Women who had a child with ASD, and who thus had an increased likelihood of having another child with ASD or other neurodevelopmental disorders given the high sibling recurrence risk for these conditions, were enrolled during a subsequent pregnancy. EARLI was implemented at four major metropolitan locations across the U.S. (Philadelphia, PA; Baltimore, MD San Francisco Bay Area, CA; and Sacramento, CA), representing three distinct US regions (Northeast, Southeast, and West). Recruitment methods varied by location to capitalize on unique resources at each study site, as previously described (Newschaffer et al. 2012). Children were then followed to age 3 years for developmental assessment. The institutional review boards (IRB) at organizations in each study site approved the EARLI study and all participants provided written informed consent.
2.2. Measurement of POP Exposures
Participants provided biological samples at any of the two study visits during the 2rd trimester or the 3rd trimester. Maternal venous blood samples were collected in trace metal-free EDTA tubes. All samples were transported to the Johns Hopkins Biological Repository for storage at −80°C, or in liquid nitrogen at −120°C. PCB congeners (n=37), PBDE congeners (n=11), and 9 persistent pesticides [hexachlorobenzene, β-hexachlorocyclohexane, γ-hexachlorocyclohexane, oxychlordane, trans-nonachlor, mirex, 2,2-bis(4-chlorophenyl)-1,1-dichloroethene (p,p´-DDE), 2,2-bis(4-chlorophenyl)-1,1,1-trichloroethane (p,p´-DDT), and 2-(4-chlorophenyl)-2-(2-chlorophenyl)-1,1,1-trichloroethane (o,p´-DDT)] were measured in maternal serum samples. Analytical determination of the target analytes was performed by gas chromatography-isotope dilution high resolution mass spectrometry (GC-IDHRMS) employing a DFS (Thermo DFS, Bremen, Germany) instrument (Jones 2012). Concentrations of target analytes were given as ng/g lipid weight (weight of serum lipids), reported as background subtracted, and corrected for the average amount present in the blank samples (Phillips et al. 1989). Three blanks were included in every set of 30 samples. Limits of detection (LOD) were calculated for each individual sample as previously described (Hamra et al. 2021; Lyall et al. 2017a). The LOD is given for every measurement and is directly dependent on the sample size used and potential interferences detected in the blank samples analyzed in parallel to unknowns. In addition, twenty women were randomly selected to have two blood samples sent off for analyses to investigate whether exposure was constant or variable with blood draw. These measures were found to be relatively stable, so only the first measure above LOD was used in subsequent statistical analyses.
2.3. Outcome Assessments
At 36 months we collected the 65-item caregiver-report Social Responsiveness Scale (SRS) (Constantino 2012), with higher raw score values indicating greater expression of the ASD-related phenotype, i.e., more deficits in social communications. Raw scores were summed individual item scores and sex-normalized T scores were also computed according to guidelines. To assess other quantitative aspects of neurodevelopmental phenotypes, we used Mullen Scales of Early Learning (MSEL) (Mullen 1995), and the Vineland Adaptive Behavior Rating Scales 2nd Edition (VABS-II) (Cicchetti 1989). Both these caregiver-report instruments were administered at 12 and 36 months. The MSEL assesses overall cognitive, language, and motor ability and the Early Learning Composite (ELC) scores shows high internal consistency and convergent validity with other measures of IQ among children. The VABS-II score is a measure of adaptive functioning of children aged 0 to 18 years and assesses the following functioning domains: communication, daily living skills, socialization, motor, and adaptive behaviors. Higher scores indicate better cognitive and adaptive skills on these instruments. Figure S1 presents total number of participants with each outcome available.
2.4. Covariate Information
In EARLI study, maternal and child demographic information were obtained through questionnaires. The following covariates were collected: maternal age, race (Black, White, other), ethnicity (Hispanic, non-Hispanic), maternal education (≤ high school graduate, college education, ≥ graduate education), annual family income (< $50,000, $50,000-$100,000, > $100,000), maternal pre-pregnancy body mass index (BMI), and child’s sex. Maternal pre-pregnancy BMI was calculated using the self-reported pre-pregnancy weight and measured height values, then further categorized into underweight (BMI<18.5 kg/m2), normal (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), and obese (>=30.0 kg/m2).
2.5. Statistical Analyses
Data from 154 participants in the EARLI study with exposure and outcome information available were included in the analytical sample. Of the 57 POP exposures measured, analytes with at least 70% of the study population above LOD were included in the final analysis (Lubin et al. 2004). A total of 11 PCBs (PCBs 28, 74, 99, 118, 138/158, 153, 170, 180, 187, 196/203), 4 BFRs (PBDEs 47, 99, 100, 153), and 2 persistent pesticides (HCB, p,p’- DDE) were analyzed. Details of the congeners included in final analysis were further described in Table S1. For participants with measurements below LOD for any of the 17 congeners, the concentration values were replaced with sample-specific LOD divided by the square root of two (Sjodin et al. 2014). Pearson correlation coefficients across the 17 congeners were calculated. Geometric mean concentrations were calculated for each congener. Given the potential non-linear relationship between the POP exposures and quantitative traits, we examined the POP concentrations as continuous natural log-transformed variables. In sensitivity analysis, we further explored the distribution of the congeners by creating a categorical variable (low exposed vs high exposed) defined by the median level of each congener and an ordinal variable split by quartiles of each biomarker concentration.
The independent effects of all 17 POPs on ASD-related phenotypes were evaluated using linear regression models with natural log-transformed POP concentration as continuous variables and quantitative traits as continuous variables, adjusting for potential confounders. We used directed acyclic graphs (DAGs) based on prior knowledge to identify potential confounders that could bias the associations between the POPs and ASD-related outcomes. Covariates were selected and retained in the adjusted model if a 15% change in coefficient estimate was observed. The final models were adjusted for study site, child’s sex, maternal education, maternal race, and maternal pre-pregnancy BMI. Missingness of covariates was relatively low (<4%) and thus complete case analysis was conducted. We also performed sensitivity analysis by including a missing category in the covariates to evaluate whether missingness influenced the results. In addition, we further adjusted for the birth year of the child, maternal smoking, and maternal age in the models for sensitivity analyses.
Next, we explored the mixture effect of POPs on the quantitative traits using the BKMR model. BKMR estimates a multivariable exposure-response function in a flexible and parsimonious way and allows for a grouped variable selection that accounts for highly correlated exposures (Bobb et al., 2018). In our analysis, we employed a hierarchical variable selection method and grouped the POP exposures into three groups-PCBs, PBDEs, and persistent pesticides. Model convergence was visually inspected using trace plots. Our grouping assumed that within-group correlation was high, and across-group correlation was low. The group posterior inclusion probability (groupPIP), representing the probability of a mixture group, and the conditional posterior inclusion probability (condPIP), representing the probability of a particular chemical within the group, were calculated. Univariate plots were used to visualize the exposure-response relationship between each POP in the given mixture while other POPs in the mixture were fixed at their 50th percentile. Potential interactions and non-linear relationships among the POPs were also visually inspected. Last, we estimated the overall joint effect of the POP mixtures on each of the traits using quantile g-computation (Keil et al. 2020). Quantile g-computation is a parametric, generalized-linear-model–based approach that uses a basic implementation of g-computation to estimate the change in each outcome for one quantile increase in all of the exposures in a given mixture. This method was implemented by first transforming all of the exposures into quantiles and then fitting a linear model between the exposure quantiles, covariates, and outcomes.
Nonlinear relationships were also explored. We examined four mixture groups-all POPs, PCBs, PBDEs, and persistent pesticides, adjusting for covariates described for the individual POP associations. Each POP exposure was assigned a weight, indicating the strength of the association between each POP exposure and the outcome. The weight is interpreted as the proportion of the negative or positive effect due to the contribution of the specific POP exposure to the mixture group. All statistical analyses were conducted using R 3.6 software.
3. Results
General characteristics of the study population (N=154) are presented in Table 1. Mothers were predominantly White (64%), non-Hispanic (83%) and had completed some college or higher education (89%). Of the 17 POP exposures included in the current analysis, 10 were detectable in ≥ 90% of the samples. The distributions of the POPs are shown in Table 2. Among all POP exposures, p,p’ DDE reported the highest concentration with a median concentration of 78.3 (IQR: 53.0–131.4) ng/g. PCB 153 had the highest concentration within PCB class (median [IQR]: 5.6 [3.6–8.7] ng/g) whereas PBDE 47 had the highest concentration within PBDE class (median 12.0 [IQR]: [5.9–19.2] ng/g). The concentrations were generally lower than the concentrations for those chemicals among women from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 cycle (Centers for Disease Control and Prevention, 2014). The correlations between the concentrations of the POP exposures are shown in Figure 1. In general, the within-class correlations were high (ranging from 0.50 to 0.98), and the across-class correlations were low (ranging from 0.02 to 0.44). PCB 28 was not strongly correlated with other PCBs (ranging from 0.06 to 0.22). HCB and p,p’ DDE were not highly correlated within persistent pesticide class as compared to PCBs and PBDEs (r=0.39). The distribution of SRS, MSEL, and VABS scores is provided in Figure S2. The distribution of child SRS raw scores showed a relatively long tail suggests presence of more individuals with higher levels of ASD-related traits. MSEL-ELC scores showed a slight shift to the left suggesting an increase in the proportion of lower cognitive abilities, while VABS scores showed a relatively normal distribution. The correlations of the quantitative traits were moderate (ranging from −0.40 to −0.57).
Table 1.
Descriptive characteristics of the study population in the Early Autism Risk Longitudinal Investigation (EARLI) study
| N (%) (n=154) |
|
|---|---|
| Maternal characteristics | |
| Maternal age, years (mean, SD) | 33.8 (4.8) |
| Maternal race | |
| White | 95 (64.2) |
| Black | 16 (10.8) |
| Other | 37 (25.0) |
| Maternal ethnicity | |
| Non-Hispanic | 127 (82.5) |
| Hispanic | 27 (17.5) |
| Maternal education | |
| High school or less | 17 (11.1) |
| Some college/college | 91 (60.0) |
| Graduate school or higher | 44 (28.9) |
| Maternal pre-pregnancy BMI | |
| Underweight or normal | 58 (39.2) |
| Overweight | 44 (29.7) |
| Obese | 46 (31.1) |
| Study site | |
| Drexel | 37 (24.0) |
| Johns Hopkins | 38 (24.6) |
| Kaiser Permanente | 46 (30.0) |
| UC Davis | 33 (21.4) |
|
Child characteristics Child’s sex |
|
| Male | 87 (56.5) |
| Female | 67 (43.5) |
| SRS raw score (mean, SD) | 37.3 (28.2) |
| MSEL ELC score (mean, SD) | 99.0 (20.5) |
| VABS composite score (mean, SD) | 93.0 (14.2) |
N (%) missing: Maternal race 6 (3.9%), Maternal education 4 (1.3%), Maternal BMI 6 (3.9%), Child SRS score 21 (13.6%), Child MSEL score 7 (4.5%), Child VABS score 21 (13.6%)
Table 2.
Distribution of POPs in maternal serum during pregnancy in the Early Autism Risk Longitudinal Investigation (EARLI) study
| Congener | %above LOD | Mean ± SD | Median (IQR) |
|---|---|---|---|
| PCBs | |||
|
| |||
| PCB 28 | 79 | 3.3 ± 3.8 | 1.8 (0.9 – 4.4) |
| PCB 74 | 83 | 1.7 ± 1.4 | 1.3 (0.9 – 2.2) |
| PCB 99 | 84 | 1.6 ± 1.2 | 1.3 (0.8 – 2.0) |
| PCB 118 | 98 | 2.9 ± 2.1 | 2.2 (1.4 – 3.7) |
| PCB 153 | 100 | 7.0 ± 5.1 | 5.6 (3.6 – 8.7) |
| PCB 138/158 | 100 | 5.6 ± 4.1 | 4.6 (2.9 – 7.0) |
| PCB 170 | 93 | 2.0 ± 1.3 | 1.6 (1.0 – 2.5) |
| PCB 180 | 100 | 4.8 ± 3.2 | 3.9 (2.6 – 6.1) |
| PCB 187 | 80 | 1.7 ± 1.6 | 1.2 (0.8 – 2.3) |
| PCB 196/203 | 77 | 1.3 ± 0.8 | 1.1 (0.7 – 1.7) |
|
| |||
| PBDEs | |||
|
| |||
| PBDE 47 | 100 | 22.4 ± 35.5 | 12.0 (5.9 – 19.2) |
| PBDE 99 | 82 | 4.9 ± 9.0 | 2.1 (1.0 – 3.7) |
| PBDE 100 | 94 | 5.2 ± 7.3 | 2.7 (1.5 – 5.8) |
| 153PBDE 153 | 99 | 12.2 ± 17.5 | 6.2 (2.9 – 13.0) |
|
| |||
| Persistent Pesticides | |||
|
| |||
| HCB | 97 | 8.0 ± 3.4 | 7.6 (6.0 – 9.3) |
| p,p’ DDE | 100 | 180.6 ± 463.5 | 78.3 (53.0 – 131.4) |
Concentrations are lipid adjusted and presented in ng/g lipids. Sample size=154
Figure 1. Pearson correlation matrix for POPs.
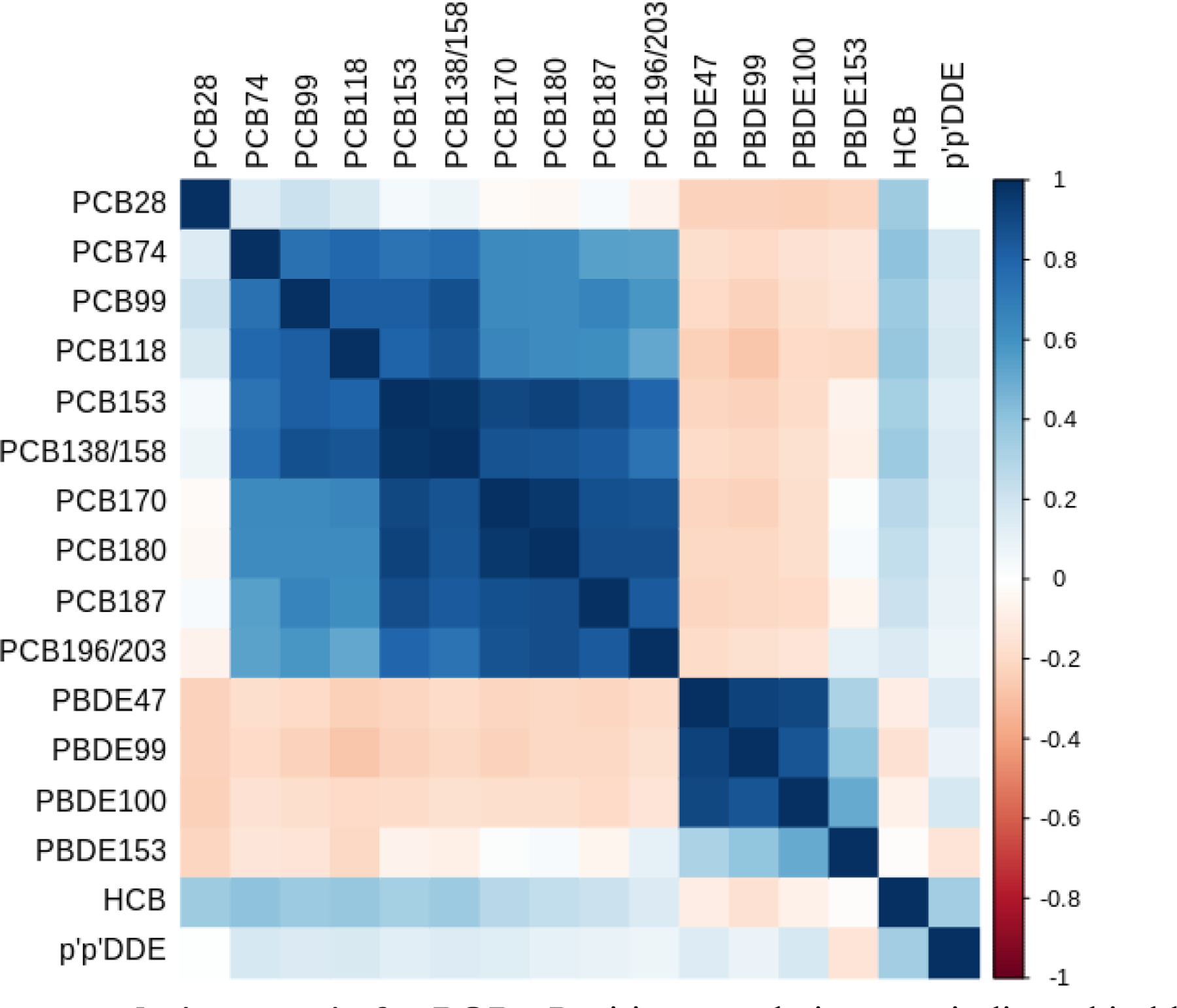
Positive correlations are indicated in blue shades and negative correlations are indicated in red shades. POPs were natural log-transformed
3.1. Association between single POP exposure and quantitative traits
Figure 2 shows the results of the independent effect of POPs and quantitative traits at 36 months from the linear regression models adjusting for confounders. We observed significantly higher SRS scores, indicating poorer social traits among participants exposed to higher ln-PBDE 47 (β=6.39, 95% CI: 1.12, 11.65, P =0.02) and higher ln-PBDE 99 (β= 5.20, 95% CI: 0.48, 9.92, P=0.03) with one-unit increase in exposure concentration (Figure 2A). In addition, maternal exposure to ln-p,p’-DDE was associated with lower SRS scores (β=−8.34, 95% CI: −15.32, −1.37, P=0.02). As shown in Figure 2B, higher PCB 180 and higher PCB 187 exposure were associated with higher MSEL-ELC scores (β=5.68, 95% CI: 0.18, 11.17, P=0.04; β=4.65, 95% CI: 0.14, 9.17, P=0.04, respectively), indicating improved cognitive functioning. We did not observe significant associations between PBDEs and pesticides and MSEL-ELC scores. Among participants with higher levels of POPs, VABS was significantly associated with PCB 170, PCB 180, PCB 187, PCB 196/203, and p,p’ DDE. For example, a one-unit increase in ln-PCB 170 was associated with a 4.6 higher VABS score at 36 months (P=0.02, Figure 2C). PBDEs were not associated with VABS scores. We did not observe significant changes to the findings after further adjustment for the birth year, smoking status, and maternal age.
Figure 2.
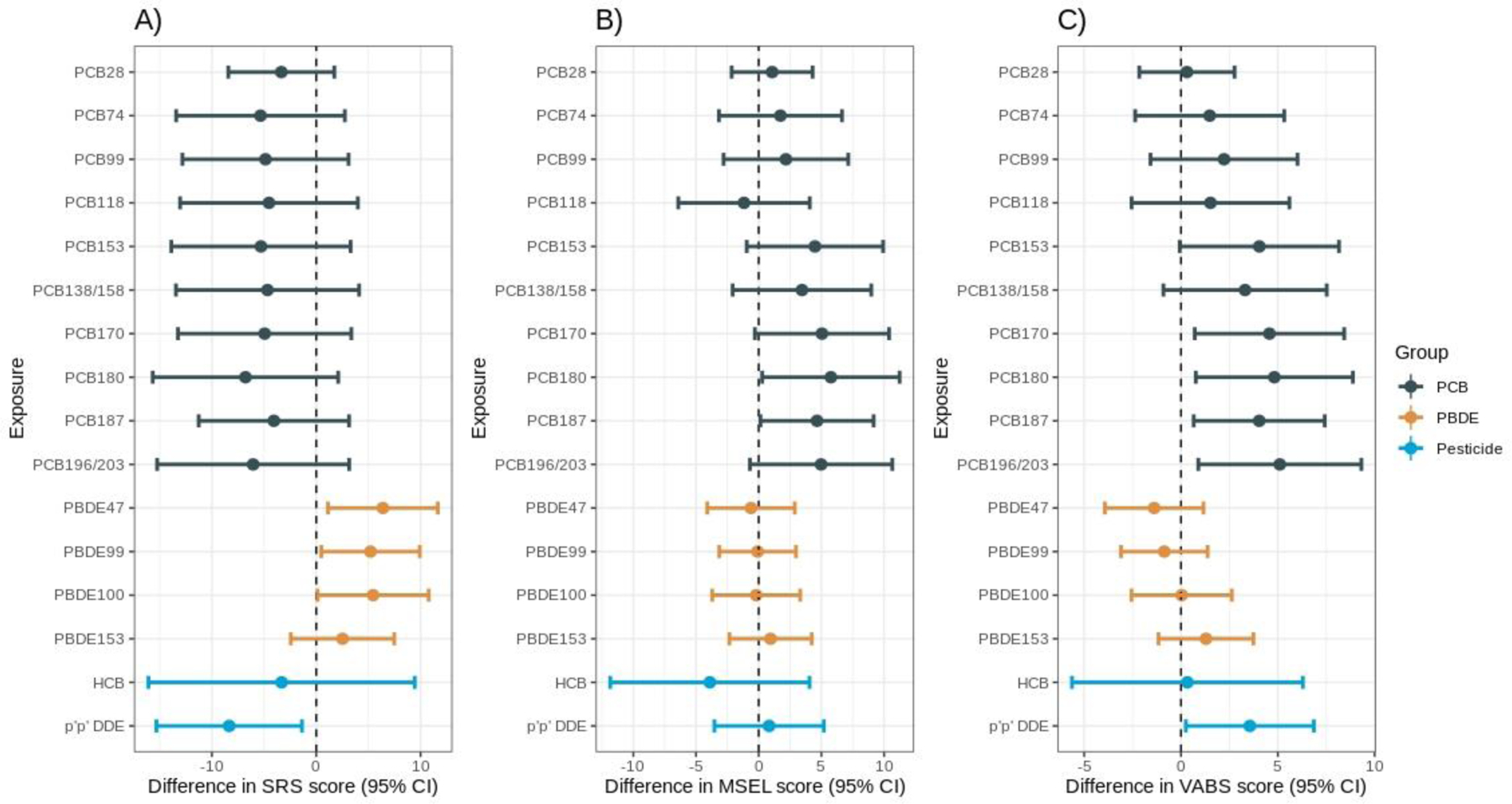
Association between single POP exposure and a) SRS score b) MSEL-ELC score c) VABS score. Estimated difference in outcome are shown for a 1-unit increase in exposures. All POP exposures were treated as continuous natural log-transformed variables. Models adjusted for study site, child’s sex, maternal education, maternal race, and maternal pre-pregnancy BMI
3.2. Association between POP mixtures and quantitative traits in BKMR model
In the BKMR analyses, although there was a linear inverse relationship between the POP mixtures and SRS score and a linear positive relationship between the POP mixtures and MSEL-ELC and VABS scores (Figure 3), these associations did not reach statistical significance. Estimated group PIPs for associations with SRS scores were 0.43, 0.58, and 0.41 for PCBs, PBDEs, and pesticides, respectively (Table S2). PCB group and PBDE group contributed more than persistent pesticides in MSEL score model (PIP=0.63, 0.71, and 0.41, respectively). In the VABS score model, PCB groups had the highest group PIP (PIP=0.62) and PCB180 contributed most within the PCB group (PIP=0.18) (Table S2). We further plotted the univariate exposure response curves for each POP, with all other POPs fixed at their median. The independent POP associations appear to be null or linear (Figure 4–5). When all other congeners were fixed at their median levels, a linear association was estimated between PBDE 47 and SRS score and an inverse association was estimated with p,p’ DDE (Figure 4). Results for PBDE 47 and p,p’ DDE were consistent with our results from independent association analysis. Increases in PCB 170 and PCB 180 were associated with higher MSEL-ELC scores, while HCB appeared to have a negative relationship (Figure 5). A weak U-shape association was identified for PBDE 99 and MSEL-ELC score (Figure 5). A positive linear association was observed between p,p’-DDE and VABS score, which was also observed in the independent association results.
Figure 3.
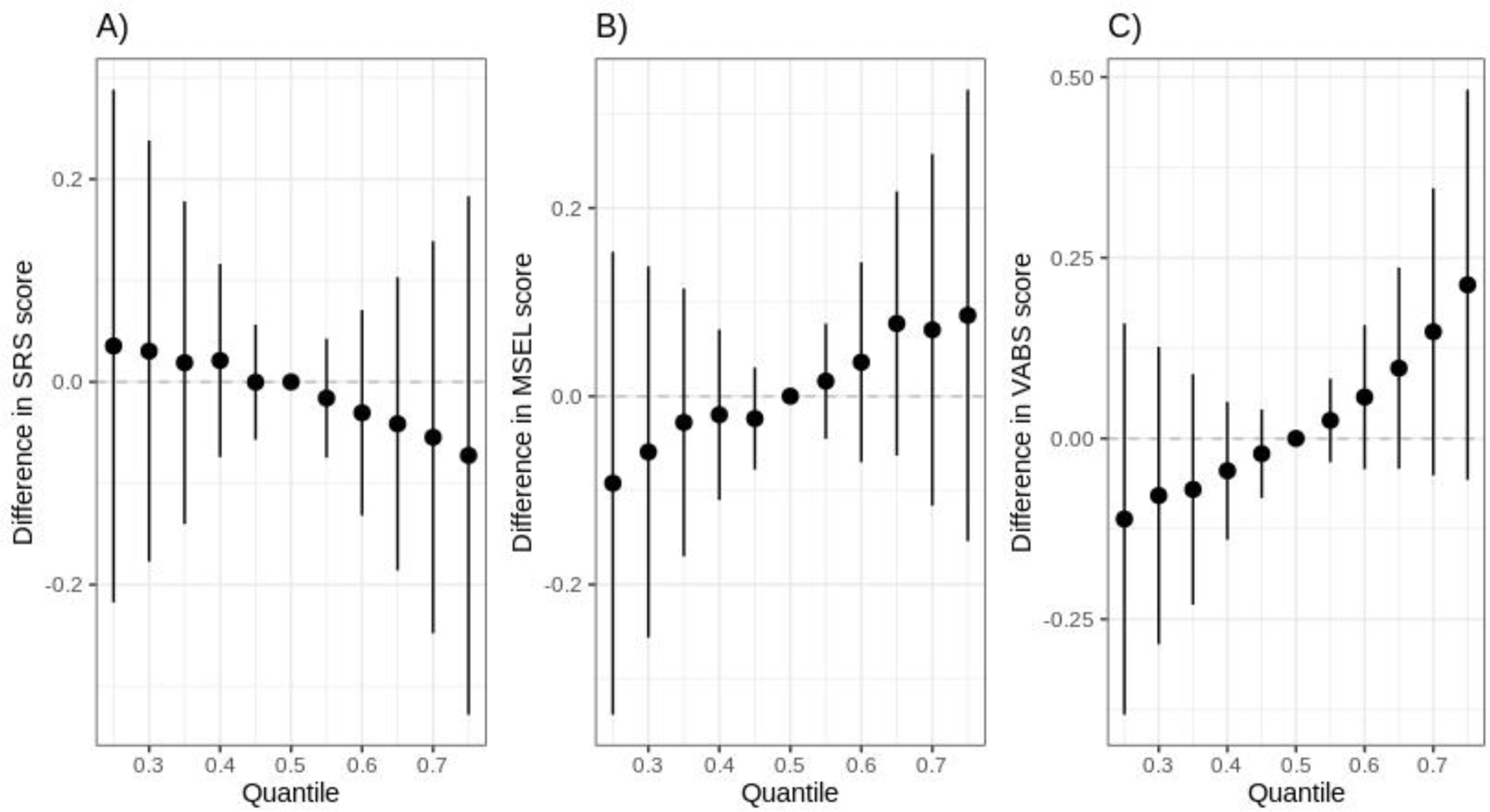
The joint effect of the POP mixtures on A) SRS score, B) MSEL-ELC score, and C) VABS score estimated from the BKMR model. Estimates and 95% confidence intervals are shown in the figure when all exposures at particular percentiles were compared to all the congeners at their 50th percentile. Models adjusted for study site, child’s sex, maternal education, maternal race, and maternal pre-pregnancy BMI
Figure 4.
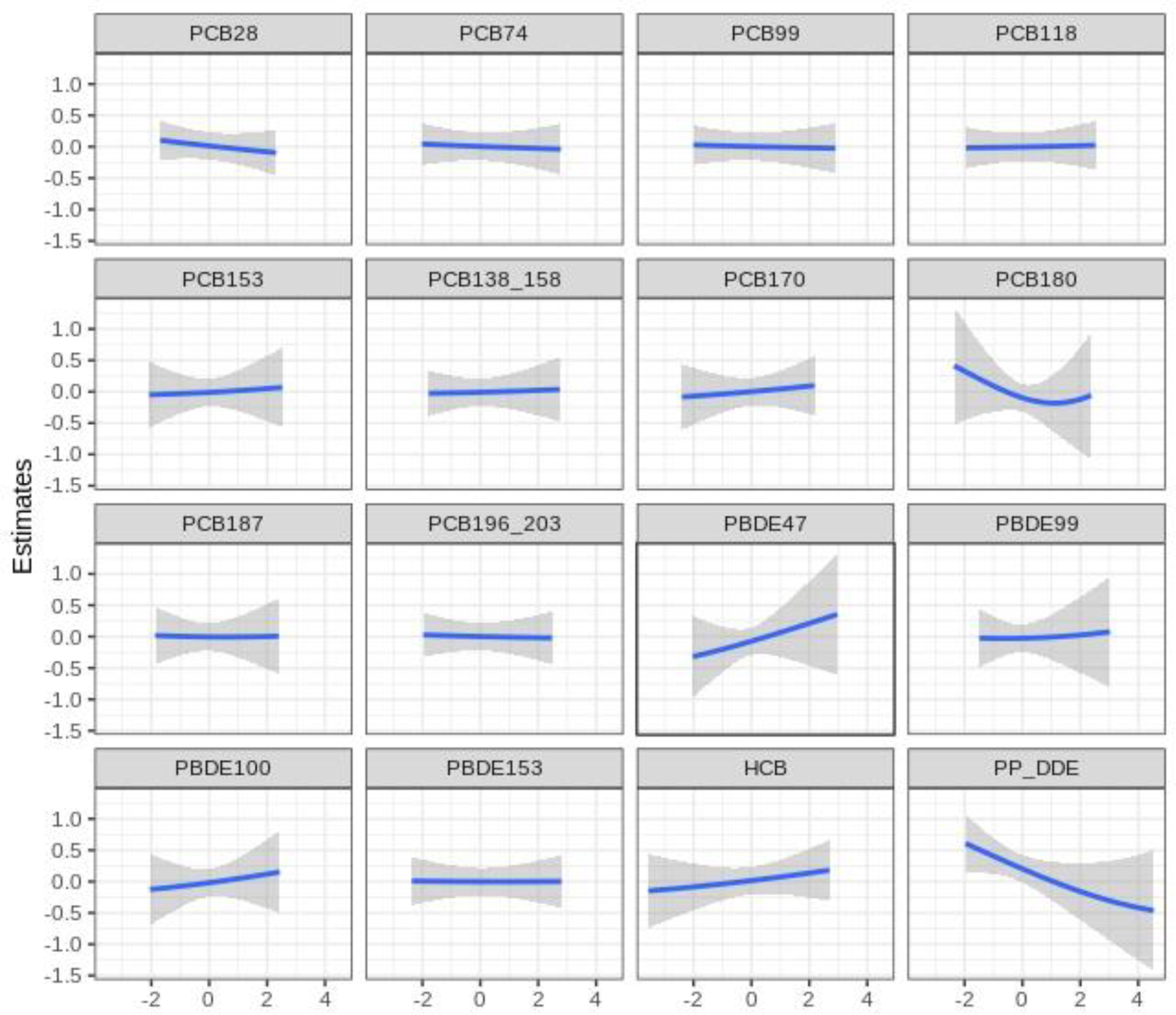
Univariate exposure-response functions for SRS score from the BKMR model. Associations between each POP and SRS score (with corresponding 95% confidence intervals) are shown when setting all other POPs at their median. Models adjusted for study site, child’s sex, maternal education, maternal race, and maternal pre-pregnancy BMI.
Figure 5.
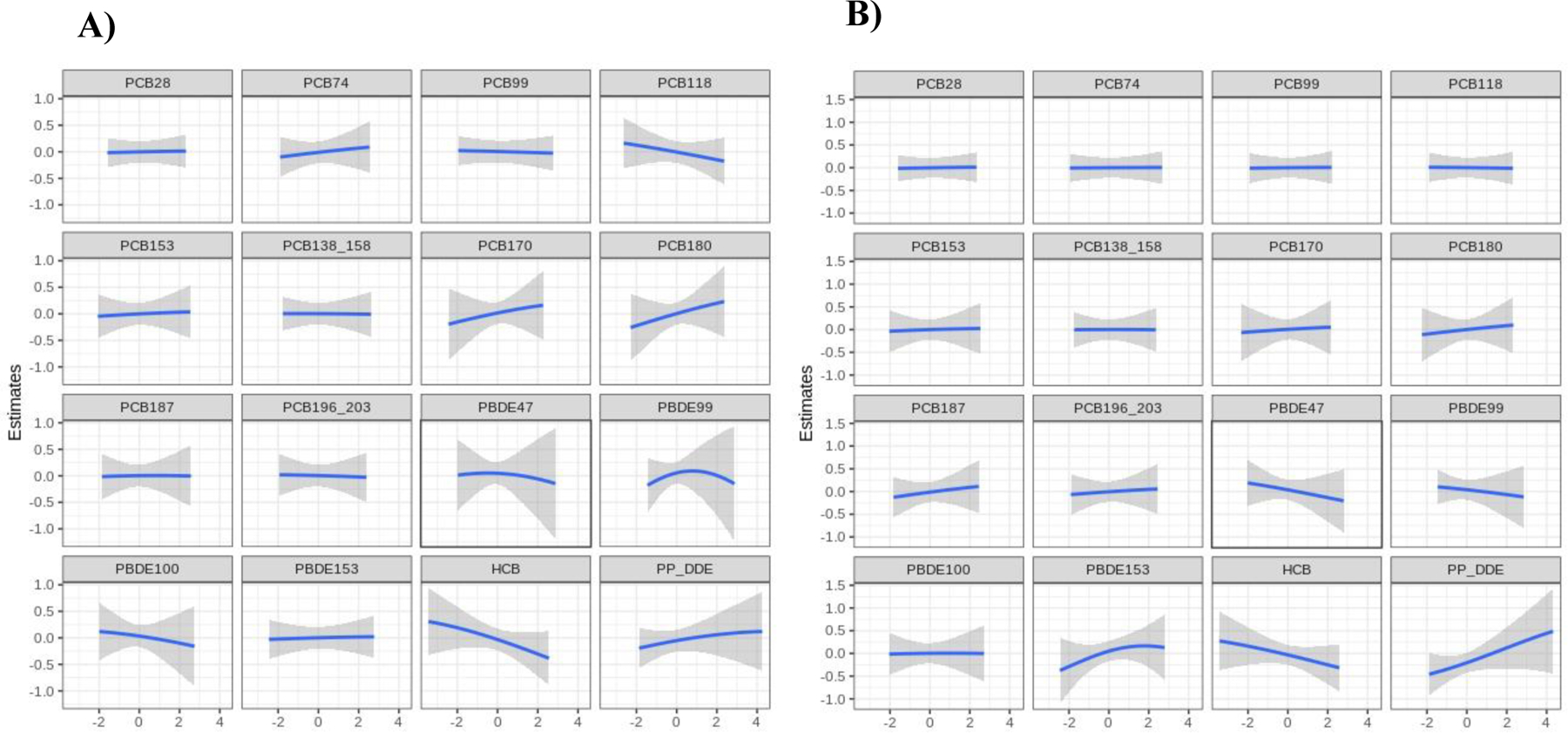
Univariate exposure-response functions for A) MSEL-ELC score and B) VABS score from the BKMR model. Associations between each POP and MSEL-ELC score (with corresponding 95% confidence intervals) are shown when setting all other POPs at their median. Models adjusted for study site, child’s sex, maternal education, maternal race, and maternal pre-pregnancy BMI.
We further estimated the contribution of individual congeners to the joint effect by only including the congeners associated with the outcomes based on the results of the univariate exposure response analysis. Figure S3-Figure S5 show the single-exposure effect of each individual congener with SRS, MSEL-ELC, and VABS scores when other congeners fixed at 25th, 50th, and 75th percentiles. Potential interactions between POPs were identified visually (Figure S6-Figure S8). As shown in Figure S9, a possible interaction was observed between PBDE 99 and MSEL-ELC score, as the PBDE99 association weakened with increasing levels of PBDE 47 and PBDE 100. We further investigated the interactions by plotting the bivariate exposure-response function and by including interaction terms in the traditional linear regression models. Significant interactions were observed between PBDE 47 and PBDE 99 (Pinteraction = 0.04) and between PBDE 100 and PBDE 99 (Pinteraction = 0.08) for the MSEL associations. We did not find evidence for interactions in the associations with SRS scores and VABS scores.
3.3. Association between POP mixtures and quantitative traits in quantile g-computation model
In the quantile g-computation analyses, we observed positive associations between POP mixtures and MSEL and VABS scores, indicating improved cognitive and adaptive skills for those with higher overall POPs in the mixture analysis (Table 3), which are consistent with those from BKMR models. Table S3 shows the results from the separate quantile g-computation models for PCBs, PBDEs, and pesticides. There was a suggestive association between the PCBs and MSEL-ELC scores (β=6.53, 95% CI: −0.67, 13.73, P=0.08), suggesting higher levels of PCBs with better cognitive functioning. PCB 187 and PBDE 47 were assigned to largest positive weight (0.26 and 0.23, respectively) whereas PCB 153 and p,p’-DDE showed the largest negative weight (−0.29 and −0.24, respectively) among the POPs for the association with SRS score. PCB 153 was identified as the driving factor for the positive association observed between the mixture and MSEL-ELC score (weight=0.43). For VABS associations, PBDE 47 had the largest weights in negative direction (weight=−0.35) and PBDE 100 showed the largest positive weight (weight=0.27) (Figure S10).
Table 3.
Association between POPs mixture and quantitative traits in quantile g-computation models
| β (95% CI) | p-value | |
|---|---|---|
| SRS score | 1.31 (−10.51, 13.14) | 0.83 |
| MSEL-ELC score | 3.25 (−4.67, 11.18) | 0.42 |
| VABS score | 3.86 (−2.30, 10.03) | 0.22 |
Estimated beta represents differences in quantitative traits per one-quantile increase in four mixture groups: all POPs, PCBs, PBDEs, persistent pesticides. Models adjusted for study site, child’s sex, maternal education, maternal race, and maternal pre-pregnancy BMI
4. Discussion
In this study, we examined associations between maternal serum POP exposures during pregnancy and ASD-related quantitative traits at 36 months in a cohort of siblings with a family history of ASD. Higher levels of PBDEs were associated with more autism-related behaviors, while higher levels of PCBs and pesticide p,p’ DDE were associated with better cognitive and adaptive functioning. No overall mixture effect of POPs on these outcomes was observed in our mixture analyses, though there was a linear trend for better behaviors and skills across all three measures: lower SRS scores (less social communication deficits associated with ASD) with higher quantile of POP mixture and higher MSEL-ELC and VABS scores (higher cognitive and adaptive functioning) with higher quantile of POP mixture.
Although there is evidence that suggests adverse associations of in utero exposure to POPs and child neurodevelopment, to date, few studies have examined the effect of POPs, both as a single-pollutant and as a mixture. In addition, the findings of those studies are heterogeneous and may relate to use of different mixture approaches, which may reveal different aspects of the associations between the mixtures and the outcomes. In a prospective birth cohort study, Braun et al. implemented a semi-Bayesian two stage approach to explore the relationship between multiple endocrine-disrupting chemicals (EDCs) and SRS scores (Braun et al. 2014). More autistic behaviors were observed with higher levels of PBDE 28 and lower autistic behaviors were observed with PCB 178 and PBDE 85 (Braun et al. 2014). In another mixture analysis, Hamra et al. applied a Bayesian approach to estimate the effect of 25 EDCs measured during pregnancy on ASD and intellectual disability and did not find evidence for an association with either diagnosis (Hamra et al. 2019). More recently, a mixture of 17 POPs were examined in association with childhood reading skills at school age in the Health Outcomes and Measures of the Environment (HOME) study utilizing six different statistical approaches (Vuong et al. 2020). Findings from the HOME study suggested an inverse relationship with prenatal exposure to PBDEs mixtures but not with all POPs. Using the same EARLI study, Hamra et al., previously reported a positive association between summed PBDEs and SRS scores and ASD risk using BWS methods (Hamra et al. 2021). The BWS allows for distinct mixture effects to be estimated for different groups of exposures and potentially identifies the contribution of individual components of the mixture to the effect, whereas the BKMR provides estimates of posterior inclusion probability for individual mixture component, meaning components with estimates of zero will be dropped from the mixture estimation model. In the present study, our analysis utilizing BKMR did not reveal an association between prenatal exposure to POP mixtures and ASD-related outcomes. Current evidence for prenatal exposure to POP mixtures and child autistic, social and cognitive-behavioral outcomes is inconclusive, but the findings of our study along with previous work highlight the importance to investigate environmental toxicants as mixtures and to consider advanced statistical approaches for future environmental epidemiological research.
We observed independent associations of PBDE 47 and PBDE 99 with more autistic behaviors and identified potential interactions between PBDE 99 and PBDE 47 / PBDE 100. The positive relationship between PBDE 47 and SRS scores was also observed in the BKMR analysis and has been previously reported using the BWS method (Hamra et al. 2021). Similar to the findings using the BWS method in the EARLI study (Hamra et al. 2021), in which PBDE47 explained more of the summed effects than other PBDEs, our mixture analysis consistently identified PBDE 47 as the driving toxicant in the PBDEs for the association with SRS score Previous studies have not shown autistic traits to be associated with PBDE 47 (Braun et al. 2014); however studies have reported that prenatal PBDE exposures are associated with neurobehavioral outcomes in children and adolescence (Dorman et al. 2018; Rossignol et al. 2014). Higher maternal serum PBDE 47 and PBDE 99 concentrations were found to be associated with decrements in cognitive abilities at age 5–8, decreases in psychomotor and mental development scores at 12, 24, and 36 months, lower IQ scores during childhood, poorer attention function at school age, and increased risk of attention-deficit/hyperactivity disorders (ADHD) (Azar et al. 2021; Braun et al. 2014; Braun et al. 2017; Chen et al. 2014; Eskenazi et al. 2013; Herbstman et al. 2010; Lenters et al. 2019; Sagiv et al. 2015). Our analysis did not confirm PBDE 47 or PBDE 99 in association with poorer cognitive scores. This present study expands upon previous work and further explored interactions between the PBDEs and other POPs. Although our statistical power for such analyses were low, to our knowledge, our study is among the first to evaluate these potential interactions in association with child neurodevelopmental outcomes. Animal studies have previously shown that interactions between PBDE 47 and PCBs and interactions between PBDE 99 and MeHg may affect long-term learning and memory function and developmental neurobehavior (Fischer et al. 2008; He et al. 2011). We were not able to explore the potential mechanisms driving the observed interactions of PBDEs in this study. Future investigation of the interactions remains an important area of research.
Research on the neurotoxicity of prenatal exposures to PCBs and persistent pesticides in animal models and epidemiological studies is growing; however, the findings of those studies are mixed. Recent reviews of epidemiological literature suggest that prenatal PCBs and p,p’ DDE were related to poorer cognitive function, more behavior problems, and increased risk of ASD (Biosca-Brull et al. 2021; Pessah et al. 2019). In another high-familial ASD likelihood study, the Markers of Autism Risk in Babies – Learning Early Signs (MARBLES) study did not find evidence for prenatal effect of POPs on risk of ASD in children (Granillo et al. 2019). In this present study, we focused on the ASD-related social, cognitive, and behavioral outcomes at 36 months. Our findings suggest a positive association of PCB 170, PCB 180, PCB 187, and PCB 196/203 with VABS scores and/or MSEL-ELC scores, indicating better cognitive and behavioral function. Two recent case-control studies have specifically studied maternal serum p,p’ DDE levels with ASD risk and reported inconsistent findings. A Finnish study found the odds of ASD increased with p,p’ DDE in the highest percentile, whereas the Early Markers for Autism (EMA) study reported a null association (Brown et al. 2018; Lyall et al. 2017a). Our results indicate less autism-related behaviors and better adaptive functioning with higher p,p’ DDE exposures. The levels of exposures, the sample size, and the age of children at outcome assessment may all contribute to the mixed findings. Moreover, the potential for confounding both in this study and other studies must be considered. For example, if postnatal exposures to POPs influenced neurodevelopment, breastfeeding would be a major source of many of these compounds and is known to benefit early development. Other nutritional factors, environmental and lifestyle factors, and maternal health conditions might also influence neurodevelopment and be associated with prenatal exposures to POPs. Further research should explore a wider range of co-factors that might confound or interact with POPs. Continued investigation in both high risk and general population settings would enhance understanding of our results and other previously reported findings. The observed associations of PCBs and p,p’ DDE in our study warrant continued investigation in both high risk and general population setting.
This is the first study to address the mixture effect of POPs on ASD-related outcomes at preschool age. Strengths of this study include prospective data collection, measurement of maternal serum POPs during gestational time periods, the application of mixture analysis approaches to account for correlated exposures, and consideration of multiple domains of child neurodevelopmental outcomes. However, several limitations should be noted. Although we adjusted for a number of potential confounders, we were not able to account for maternal cognitive function or maternal psychological conditions, which may introduce unmeasured confounding. Pesticides, air pollution, phthalates, and metals have been associated with ASD or ASD traits and were not adjusted in these analyses. Additionally, nutrition, as well as breastfeeding that could result in high post-natal exposures were not accounted for. The EARLI study recruited participants from four regions in the US and the levels of POPs in the EARLI study were slightly lower than the reported levels in the NHANES study. This may reduce the generalizability to some other regions. Because of the small sample size, we were not able to further explore modification by child sex or race. Future work should evaluate effect modification in a larger sample population.
We note that our results may appear paradoxical, in suggesting that increased exposure to some POPs measured during pregnancy may be associated with improved neurodevelopmental outcomes. Such findings may be partially driven by the unique selection of EARLI, which requires an older sibling with ASD by design, resulting in not only increased rates of ASD and developmental delay diagnoses but also shifts in quantitative trait distributions away from general population norms. Common sources of PCBs, PBDEs, and p,p’ DDE are largely dietary in nature and thought to come from consumption of fish, meat, and dairy products. Given the higher level of education and associated socio-economic status in EARLI, combined with having had a child with ASD, such routes of exposure may be elevated in our population as these same foods are known to contain nutrients (e.g. PUFAs, iron) which foster brain development. Future research would merit from investigation of such chemicals in the context of the broader diet and in conjunction with other nutrients. Finally, while results on POPs and neurodevelopmental traits have been mixed, some studies have also identified directions of effect consistent with our own (Braun et al. 2014; Caspersen et al. 2016; Neugebauer et al. 2015; Nowack et al. 2015).
Our study investigated the independent and joint effects of prenatal exposure to POPs and autism-related social, cognitive, and behavioral outcomes in children. Our findings suggest independent associations of several PCBs, PBDEs, and p,p’ DDE with the quantitative traits. There was no significant association between POP mixtures and the neurodevelopmental outcomes. Future research is needed to evaluate mixture effects and interaction of POPs in other study populations whose background risks and component chemicals may differ and represent varying sources and duration of exposure to POPs.
Supplementary Material
Highlights.
The associations between prenatal exposure to POPs mixtures and autism-related traits in children were evaluated
Prenatal exposure to PCBs, PBDEs, and p,p’ DDE were associated with autism-related traits at 36 months of age
Potential interactions were identified between PBDE 99 and other PBDEs.
Acknowledgements
We like to thank the EARLI families that participated in the study and made this work possible. This work was supported through grants from the National Institutes of Health (NIH) to Craig Newschaffer (R01ES016443, R01ES026903), Heather Volk (R24ES030893).
Abbreviations:
- POP
persistent organic pollutant
- ASD
autism spectrum disorder
- PCB
polychlorinated biphenyl
- PBDE
polybrominated diphenyl ethers
- HCB
hexachlorobenzene
- p,p´-,DDE
p,p´-dichlorodiphenyldichloroethene
- LOD
limit of detection
- SRS
Social Responsiveness Scale scores
- MSEL-ELC
Mullen Scales of Early Learning Early Learning Composite scores
- VABS
Vineland Adaptive Behavior Scales
- BMI
body mass index
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Credit Author Statement
CN, LC, IH-P, and MF developed the cohort. HV and AS conceptualized and designed the project. MF and HV funded and supervised the project. AS and EK performed the analyses and code review. AS and HV drafted the manuscript. GH, AD, LC, IH-P, RS, and MF provided the manuscript revisions and editing. All authors reviewed the results and approved the submitted version of the manuscript.
Availability of data
The dataset can be found in the repository: The National Institute of Mental Health Data Archive (NDA), under the collections for the EARLI study (1600) https://nda.nih.gov/edit_collection.html?id=1600.
Conflict of Interest
The authors declare they have no conflict of interest
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ashwood P, Schauer J, Pessah IN, Van de Water J. 2009. Preliminary evidence of the in vitro effects of bde-47 on innate immune responses in children with autism spectrum disorders. J Neuroimmunol 208:130–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azar N, Booij L, Muckle G, Arbuckle TE, Seguin JR, Asztalos E, et al. 2021. Prenatal exposure to polybrominated diphenyl ethers (pbdes) and cognitive ability in early childhood. Environ Int 146:106296. [DOI] [PubMed] [Google Scholar]
- Biosca-Brull J, Perez-Fernandez C, Mora S, Carrillo B, Pinos H, Conejo NM, et al. 2021. Relationship between autism spectrum disorder and pesticides: A systematic review of human and preclinical models. Int J Environ Res Public Health 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobb JF, Claus Henn B, Valeri L, B.A. Coull Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression Environ. Health, 17 (1) (2018. Aug 20), p. 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kalkbrenner AE, Just AC, Yolton K, Calafat AM, Sjodin A, et al. 2014. Gestational exposure to endocrine-disrupting chemicals and reciprocal social, repetitive, and stereotypic behaviors in 4- and 5-year-old children: The home study. Environ Health Perspect 122:513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Yolton K, Stacy SL, Erar B, Papandonatos GD, Bellinger DC, et al. 2017. Prenatal environmental chemical exposures and longitudinal patterns of child neurobehavior. Neurotoxicology 62:192–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Cheslack-Postava K, Rantakokko P, Kiviranta H, Hinkka-Yli-Salomaki S, McKeague IW, et al. 2018. Association of maternal insecticide levels with autism in offspring from a national birth cohort. Am J Psychiatry 175:1094–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspersen IH, Aase H, Biele G, Brantsaeter AL, Haugen M, Kvalem HE, et al. 2016. The influence of maternal dietary exposure to dioxins and pcbs during pregnancy on adhd symptoms and cognitive functions in norwegian preschool children. Environ Int 94:649–660. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2014. Fourth National Report on HumanExposure to Environmental Chemicals Updated Tables. NationalCenter for Environmental Health, Centers for Disease Controland Prevention http://www.cdc.gov/exposurereport [accessed 28 April 2023].
- Chen A, Yolton K, Rauch SA, Webster GM, Hornung R, Sjodin A, et al. 2014. Prenatal polybrominated diphenyl ether exposures and neurodevelopment in u.S. Children through 5 years of age: The home study. Environ Health Perspect 122:856–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Sjodin A, McLachlan MS, English K, Aylward LL, Toms LL, et al. 2017. Persistent organic pollutants in infants and toddlers: Relationship between concentrations in matched plasma and faecal samples. Environ Int 107:82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheslack-Postava K, Rantakokko PV, Hinkka-Yli-Salomaki S, Surcel HM, McKeague IW, Kiviranta HA, et al. 2013. Maternal serum persistent organic pollutants in the finnish prenatal study of autism: A pilot study. Neurotoxicol Teratol 38:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti SSSDV. 1989. The vineland adaptive behavior scales. In: Major psychological assessment instruments, Vol. 2:Allyn & Bacon, 199–231. [Google Scholar]
- Constantino JN. 2012. Social responsiveness scale, second edition (srs-2) [manual]. Torrance, CA:Western Psychological Services. [Google Scholar]
- de Escobar GM, Obregon MJ, del Rey FE. 2004. Maternal thyroid hormones early in pregnancy and fetal brain development. Best Pract Res Clin Endocrinol Metab 18:225–248. [DOI] [PubMed] [Google Scholar]
- Dorman DC, Chiu W, Hales BF, Hauser R, Johnson KJ, Mantus E, et al. 2018. Polybrominated diphenyl ether (pbde) neurotoxicity: A systematic review and meta-analysis of animal evidence. J Toxicol Environ Health B Crit Rev 21:269–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunaway KW, Islam MS, Coulson RL, Lopez SJ, Vogel Ciernia A, Chu RG, et al. 2016. Cumulative impact of polychlorinated biphenyl and large chromosomal duplications on DNA methylation, chromatin, and expression of autism candidate genes. Cell Rep 17:3035–3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Chevrier J, Rauch SA, Kogut K, Harley KG, Johnson C, et al. 2013. In utero and childhood polybrominated diphenyl ether (pbde) exposures and neurodevelopment in the chamacos study. Environ Health Perspect 121:257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer C, Fredriksson A, Eriksson P. 2008. Coexposure of neonatal mice to a flame retardant pbde 99 (2,2’,4,4’,5-pentabromodiphenyl ether) and methyl mercury enhances developmental neurotoxic defects. Toxicol Sci 101:275–285. [DOI] [PubMed] [Google Scholar]
- Gaugler T, Klei L, Sanders SJ, Bodea CA, Goldberg AP, Lee AB, et al. 2014. Most genetic risk for autism resides with common variation. Nat Genet 46:881–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granillo L, Sethi S, Keil KP, Lin Y, Ozonoff S, Iosif AM, et al. 2019. Polychlorinated biphenyls influence on autism spectrum disorder risk in the marbles cohort. Environ Res 171:177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove J, Ripke S, Als TD, Mattheisen M, Walters RK, Won H, et al. 2019. Identification of common genetic risk variants for autism spectrum disorder. Nat Genet 51:431–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamra GB, Lyall K, Windham GC, Calafat AM, Sjodin A, Volk H, et al. 2019. Prenatal exposure to endocrine-disrupting chemicals in relation to autism spectrum disorder and intellectual disability. Epidemiology 30:418–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamra GB, Maclehose RF, Croen L, Kauffman EM, Newschaffer C. 2021. Bayesian weighted sums: A flexible approach to estimate summed mixture effects. Int J Environ Res Public Health 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He P, Wang A, Niu Q, Guo L, Xia T, Chen X. 2011. Toxic effect of pbde-47 on thyroid development, learning, and memory, and the interaction between pbde-47 and pcb153 that enhances toxicity in rats. Toxicol Ind Health 27:279–288. [DOI] [PubMed] [Google Scholar]
- Herbstman JB, Sjodin A, Kurzon M, Lederman SA, Jones RS, Rauh V, et al. 2010. Prenatal exposure to pbdes and neurodevelopment. Environ Health Perspect 118:712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Park HY, Dostal M, Kocan A, Trnovec T, Sram R. 2008. Prenatal exposures to persistent and non-persistent organic compounds and effects on immune system development. Basic Clin Pharmacol Toxicol 102:146–154. [DOI] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Schmidt RJ, Krakowiak P. 2018. Understanding environmental contributions to autism: Causal concepts and the state of science. Autism Res 11:554–586. [DOI] [PubMed] [Google Scholar]
- Iossifov I, O’Roak BJ, Sanders SJ, Ronemus M, Krumm N, Levy D, et al. 2014. The contribution of de novo coding mutations to autism spectrum disorder. Nature 515:216–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RE E; Anderson S; Zhang Y; Sjodin A 2012. Semi-automated extraction and cleanup method for measuring persistent organic pollutants in human serum. Organohalogen Compounds:97–98.
- Keil AP, Buckley JP, O’Brien KM, Ferguson KK, Zhao S, White AJ. 2020. A quantile-based g-computation approach to addressing the effects of exposure mixtures. Environ Health Perspect 128:47004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufer BI, Neier K, Valenzuela AE, Yasui DH, Schmidt RJ, Lein PJ, et al. 2022. Placenta and fetal brain share a neurodevelopmental disorder DNA methylation profile in a mouse model of prenatal pcb exposure. Cell Rep 38:110442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenters V, Iszatt N, Forns J, Cechova E, Kocan A, Legler J, et al. 2019. Early-life exposure to persistent organic pollutants (ocps, pbdes, pcbs, pfass) and attention-deficit/hyperactivity disorder: A multi-pollutant analysis of a norwegian birth cohort. Environ Int 125:33–42. [DOI] [PubMed] [Google Scholar]
- Liu C, Wang C, Yan M, Quan C, Zhou J, Yang K. 2012. Pcb153 disrupts thyroid hormone homeostasis by affecting its biosynthesis, biotransformation, feedback regulation, and metabolism. Horm Metab Res 44:662–669. [DOI] [PubMed] [Google Scholar]
- Lubin JH, Colt JS, Camann D, Davis S, Cerhan JR, Severson RK, et al. 2004. Epidemiologic evaluation of measurement data in the presence of detection limits. Environ Health Perspect 112:1691–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall K, Croen LA, Sjodin A, Yoshida CK, Zerbo O, Kharrazi M, et al. 2017a. Polychlorinated biphenyl and organochlorine pesticide concentrations in maternal mid-pregnancy serum samples: Association with autism spectrum disorder and intellectual disability. Environ Health Perspect 125:474–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall K, Croen LA, Weiss LA, Kharrazi M, Traglia M, Delorenze GN, et al. 2017b. Prenatal serum concentrations of brominated flame retardants and autism spectrum disorder and intellectual disability in the early markers of autism study: A population-based case-control study in california. Environ Health Perspect 125:087023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maenner MJ, Shaw KA, Bakian AV, Bilder DA, Durkin MS, Esler A, et al. 2021. Prevalence and characteristics of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, united states, 2018. MMWR Surveill Summ 70:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen EM. 1995. Mullen scales of early learning (ags ed.). Circle Pines, MN:American Guidance Service Inc. [Google Scholar]
- Neugebauer J, Wittsiepe J, Kasper-Sonnenberg M, Schoneck N, Scholmerich A, Wilhelm M. 2015. The influence of low level pre- and perinatal exposure to pcdd/fs, pcbs, and lead on attention performance and attention-related behavior among german school-aged children: Results from the duisburg birth cohort study. Int J Hyg Environ Health 218:153–162. [DOI] [PubMed] [Google Scholar]
- Newschaffer CJ, Croen LA, Fallin MD, Hertz-Picciotto I, Nguyen DV, Lee NL, et al. 2012. Infant siblings and the investigation of autism risk factors. J Neurodev Disord 4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowack N, Wittsiepe J, Kasper-Sonnenberg M, Wilhelm M, Scholmerich A. 2015. Influence of low-level prenatal exposure to pcdd/fs and pcbs on empathizing, systemizing and autistic traits: Results from the duisburg birth cohort study. PLoS One 10:e0129906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessah IN, Lein PJ, Seegal RF, Sagiv SK. 2019. Neurotoxicity of polychlorinated biphenyls and related organohalogens. Acta Neuropathol 138:363–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips DL, Pirkle JL, Burse VW, Bernert JTLO Jr., Needham LL 1989. Chlorinated hydrocarbon levels in human serum: Effects of fasting and feeding. Arch Environ Contam Toxicol 18:495–500. [DOI] [PubMed] [Google Scholar]
- Rossignol DA, Genuis SJ, Frye RE. 2014. Environmental toxicants and autism spectrum disorders: A systematic review. Transl Psychiatry 4:e360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagiv SK, Kogut K, Gaspar FW, Gunier RB, Harley KG, Parra K, et al. 2015. Prenatal and childhood polybrominated diphenyl ether (pbde) exposure and attention and executive function at 9–12 years of age. Neurotoxicol Teratol 52:151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjodin A, Jones RS, Caudill SP, Wong LY, Turner WE, Calafat AM. 2014. Polybrominated diphenyl ethers and other persistent organic pollutants in serum pools from the national health and nutrition examination survey: 2001–2002. Environ Sci Technol Lett 1:92–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson MR, Boekelheide K. 2013. Multiple environmental chemical exposures to lead, mercury and polychlorinated biphenyls among childbearing-aged women (nhanes 1999–2004): Body burden and risk factors. Environ Res 121:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong AM, Xie C, Jandarov R, Dietrich KN, Zhang H, Sjodin A, et al. 2020. Prenatal exposure to a mixture of persistent organic pollutants (pops) and child reading skills at school age. Int J Hyg Environ Health 228:113527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye BS, Leung AOW, Wong MH. 2017. The association of environmental toxicants and autism spectrum disorders in children. Environ Pollut 227:234–242. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


