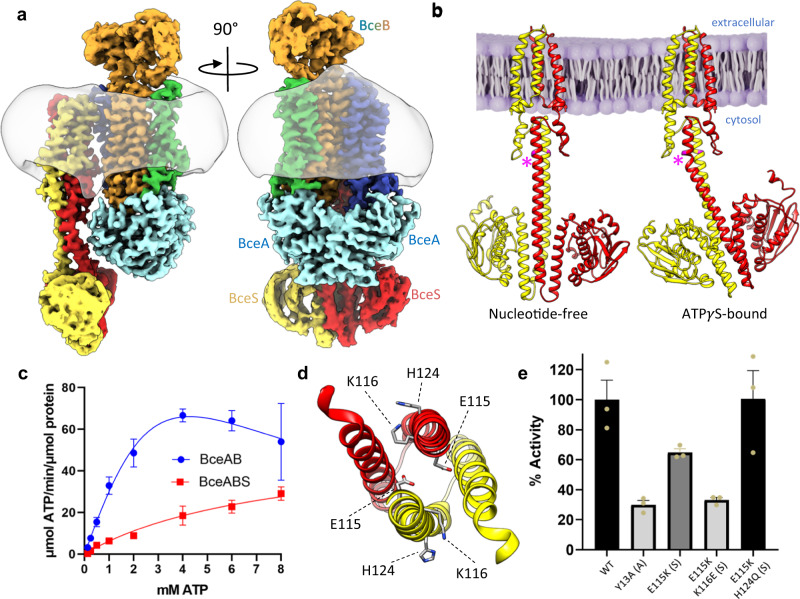
Fig. 5. ATPγS binding induces an intermediate conformation of BceAB-S.
a Two rotated views of the cryo-EM map of BceAB-S bound to ATPγS. Individual protein chains and TM helices are colored as indicated in Fig. 1. The detergent micelle is shown in transparent grey. Binding of ATPγS causes the BceA subunits (cyan) to collapse into a more tightly closed and symmetrical dimer. b Comparison of BceS configurations in nucleotide-free and ATPγS bound states of the BceAB-S complex. Binding of ATPγS to BceAB-S induces a ~ 30° kink in the stalk helix of one BceS monomer (pink asterisk). c ATPase measurements of detergent solubilized BceAB and BceAB-S complexes. Isolated BceAB exhibits higher basal ATPase activity than the complex with the BceS sensor kinase. Data points represent the mean across n = 3 triplicate measurements, and error bars represent standard deviation (SD) across the three measurements. d View of the DHp domain of BceS showing H124 that is auto-phosphorylated, and residues E115 and K116 that when substituted to charged-swap variants produce a constitutively active BceS in B. subtilis. e Comparison of maximal ATPase activity for WT and charge swap variants of the BceAB-S complex. Error bars represent standard error of the mean (SEM) across n = 3 triplicate measurements (shown in tan spheres).