Abstract
Background:
In the current consensus criteria, onset after age 75 is considered as non-supporting for diagnosis of multiples system atrophy (MSA); however, some MSA patients present after age 75. Clinical and pathological characteristics of such later onset MSA (LO-MSA) compared to usual onset MSA (UO-MSA) remain poorly understood.
Methods:
The clinical cohort included patients from Kobe University Hospital and Amagasaki General Medical Center Hospital, while the autopsy cohort was from the brain bank at Mayo Clinic Florida. We identified 83 patients in the clinical cohort and 193 patients in the autopsy cohort. We divided MSA into two groups according to age at onset: UO-MSA (≤ 75) and LO-MSA (> 75). We compared clinical features and outcomes between the two groups in the clinical cohort and compared the findings to the autopsy cohort.
Results:
LO-MSA accounted for 8% in the clinical cohort and 5% in the autopsy cohort. The median time from onset to death or to life-saving tracheostomy was significantly shorter in LO-MSA than in UO-MSA in both cohorts (4.8 vs 7.9 years in the clinical cohort and 3.9 vs 7.5 years in the autopsy cohort; P = 0.043 and P < 0.0001, respectively). The median time from diagnosis to death was less than 3 years in LO-MSA in the clinical cohort.
Conclusions:
Some MSA patients have late age of onset and short survival, limiting time for clinical decision making. MSA should be considered in the differential diagnosis of elderly patients with autonomic symptoms and extrapyramidal and/or cerebellar syndromes.
Introduction
Multiple system atrophy (MSA) is a fatal adult-onset neurodegenerative disease characterized by varying degrees of cerebellar dysfunction, Parkinsonism, and autonomic failure [7]. MSA is classified as an α-synucleinopathy due to pathological α-synuclein aggregates in glial cells [22, 27] and neurons [6, 30]. The prognosis of MSA is poor, with the median survival between 6 and 10 years [1, 5, 8, 21, 24, 37, 39, 40]. A previous study reported that the average age at onset was 53 years [39]. The second consensus statement of MSA published in 2008 has been widely used in the diagnosis of MSA [9]. In these criteria, onset after age 75 is considered a non-supporting feature; however, in clinical practice, MSA patients may present with initial signs and symptoms after age 75 [14, 19, 28]. The clinical characteristics of later onset (LO) MSA remain poorly understood. An accurate diagnosis of LO-MSA is challenging because the diagnosis is based on clinical features without reliable biomarkers and several MSA mimics exist [11, 13, 18, 26]. We defined LO-MSA as patients with onset of clinical features after 75 years of age. We aimed to describe clinical characteristics of LO-MSA to improve its recognition and differentiation from other late onset neurodegenerative disorders. In addition to a report of LO-MSA from Asia [19], the presence of LO-MSA has been suggested in an autopsy cohort from the United States [14]. In this study, therefore, we used a retrospective clinical cohort from Japan and an autopsy cohort from the United States to examine clinical and pathological features of LO-MSA.
Methods
Study cohorts
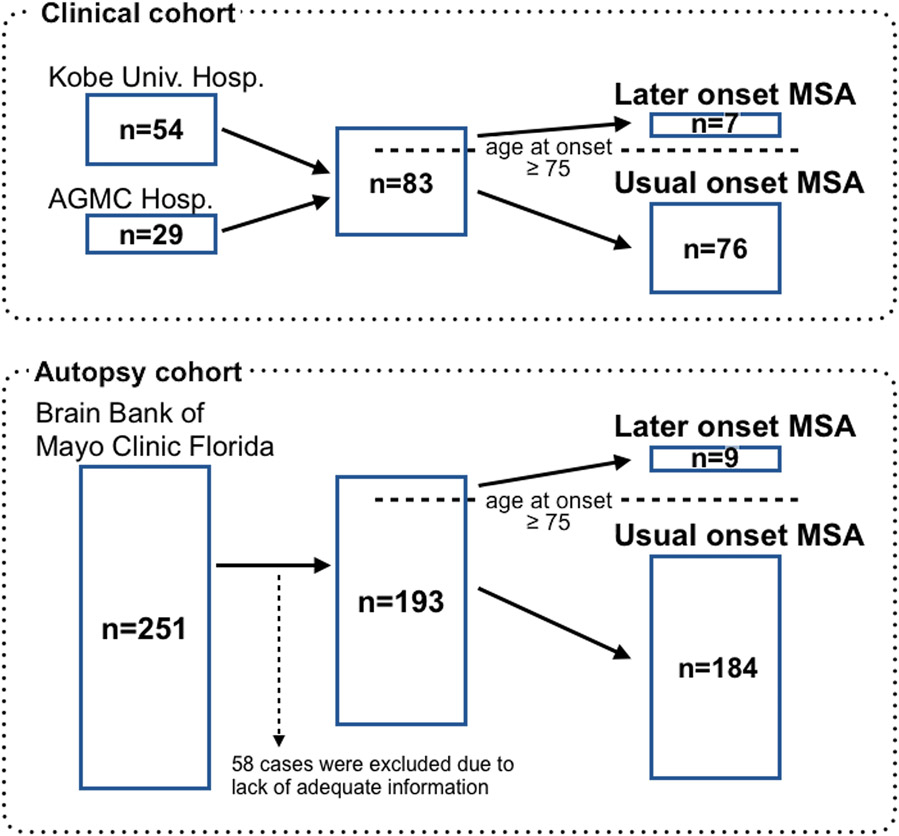
We used two independent MSA cohorts in this study: a clinical cohort and an autopsy cohort (Figure 1). For the clinical cohort, we reviewed medical records of 54 consecutive MSA patients admitted to Kobe University Hospital (Kobe, Japan) and 29 consecutive MSA patients admitted to Hyogo Prefectural Amagasaki General Medical Center Hospital (Amagasaki, Japan) between 2015 and 2019. In the clinical cohort, a diagnosis of MSA was made if the patient met criteria for possible MSA or probable MSA according to the Gilman criteria [9], except for the age of onset. At the time of diagnosis, all clinical cases were hospitalized and evaluated by multiple board-certificated neurologists.
Figure 1. Study design.
We included 83 MSA patients in the clinical cohort. We identified 251 MSA patients in the Mayo Clinic Brain Bank. Fifty-eight patients were excluded from the present study because they lack adequate clinical information to identify the symptom onset and initial symptoms. The number of LO-MSA patients was 7 in the clinical cohort and 9 in the autopsy cohort.
MSA, multiple system atrophy; AGMC, Amagasaki General Medical Center; Hosp., Hospital; LO, later onset
We compared the results in the clinical cohort with pathologically confirmed MSA from the Mayo Clinic brain bank. We identified 251 consecutive MSA patients in the brain bank between 1998 and 2018. We excluded 58 cases from the study because there was not enough clinical information to identify the time of onset or the initial symptoms. As a result, 193 MSA patients were examined in the autopsy cohort. All autopsy cases underwent a systematic and standardized neuropathologic assessment. Information from 83 patients was previously reported in a study on diagnostic pitfalls of MSA [13], and information from 171 patients was included in a study describing clinical features of autopsy-confirmed MSA [14].
We divided MSA cases in both clinical and autopsy cohorts into two groups according to the age of symptomatic onset: usual onset (≤ 75 years; UO-MSA) and later onset (> 75 years; LO-MSA). We compared the clinical features and outcomes between the two groups.
Clinical information
We systematically reviewed medical records of each patient. In pathologically confirmed cases, we also abstracted data from brain bank questionnaires filled out by family members. We collected the following clinical information: sex, race, age at onset, initial symptoms, age at death, age of tracheostomy in a life-threatening situation, and clinical subtype (MSA-C or MSA-P). The prognosis of each group was examined based on the interval from the onset to death or onset to non-preventive tracheostomy in a life-threatening situation of respiratory failure caused by severe pneumonia, asphyxiation, and vocal cord paralysis. In the clinical cohort, the diagnosis was made after a thorough inpatient examination, which allowed us to assess the date of initial diagnosis and examine the interval from onset to diagnosis and from diagnosis to death or from diagnosis to tracheostomy. We also ascertained initial symptoms of each case from the medical records in both cohorts. The initial symptoms include motor symptoms (gait difficulty, gait ataxia, speech problem, bradykinesia, tremor, hand clumsiness, frequent falls, dystonia, apraxia, and cramp) and non-motor symptoms (frequent urination, urinary incontinence, urinary retention, orthostatic hypotension, dizziness, erectile dysfunction, cognitive impairment, pain, paraesthesia, and stridor). If multiple symptoms were present at initial presentation, they were described separately. In the clinical cohort, we investigated clinical signs and symptoms at the time of diagnosis of MSA. They include cerebellar ataxia, extrapyramidal symptoms, pyramidal signs, orthostatic hypotension, urinary problems, cognitive impairment, and downgaze palsy. All MSA patients underwent magnetic resonance imaging (MRI) of the brain at least one time (63% of patients underwent more than one scan). This enabled us to review MRI findings suggestive of MSA in the clinical cohort. We considered the following abnormalities to be suggestive of MSA: “hot cross bun” sign, hyperintense putaminal rim sign, atrophy of putamen, atrophy of pons, and atrophy of middle cerebellar peduncle for both MSA-C and MSA-P and atrophy of cerebellum for MSA-P [4, 9]. In the autopsy cohort, we also investigated the initial and final clinical diagnoses.
Neuropathological assessments
Autopsy-confirmed MSA cases were evaluated with a standardized neuropathologic assessment as previously reported [13]. Paraffin-embedded sections were studied with hematoxylin & eosin staining and with thioflavin S fluorescent microscopy, as well as immunohistochemistry with an antibody against αSYN (NACP, rabbit polyclonal, 1:3000, with formic acid pretreatment). Braak neurofibrillary tangle (NFT) stage [3] and Thal amyloid phase [33] were assigned by thioflavin S fluorescent microscopy. A pathological diagnosis of MSA was established based on widespread and abundant GCIs with neurodegeneration in the striatonigral or olivopontocerebellar systems, or both [16, 35]. All cases were evaluated by a board-certified neuropathologist (DWD). We assigned pathological subtypes of MSA based on the severity of affected regions: striatonigral degeneration (MSA-SND) had most severe pathology in striatonigral regions, olivopontocerebellar atrophy (MSA-OPCA) had most severe pathology in olivopontocerebellar regions, and MSA-SND/OPCA had these regions equally affected. We also defined “minimal change” MSA as no or minimal neuronal loss and gliosis in striatonigral and olivopontocerebellar systems despite widespread distribution of GCIs [15, 17, 20, 36, 41].
Statistical analysis
We conducted statistical analysis with GraphPad Prism (version 9.1.2, GraphPad Software, La Jolla, CA, USA). Comparison of the probability of survival between the LO-MSA group and the UO-MSA group was performed by Kaplan-Meier survival analysis. Statistical significance of Kaplan-Meier survival curves was determined with the Gehan-Breslow-Wilcoxon test. We conducted the Mann-Whitney test to compare the time from onset to diagnosis between LO-MSA and UO-MSA in the clinical cohort. We used Fisher’s exact test to compare categorical variables between LO-MSA and UO-MSA. To compare the distribution of pathological subtypes between LO-MSA and UO-MSA, we conducted Fisher’s exact test with the Bonferroni correction. We compared the diagnostic accuracy among pathological subtypes (MSA-SND vs. MSA-OPCA vs. MSA-mixed) by the chi-square test. The correlation between disease duration and the Braak NFT stage or Thal amyloid phase was examined by Spearman’s correlation analysis. Statistical significance was defined by a p-value < 0.05.
Results
Clinical cohort
We identified 7 LO-MSA and 76 UO-MSA in the clinical cohort. The frequency of LO-MSA was 8%. We summarize the characteristics of each group in Table 1. The mean age of onset was 78 ± 3 years in LO-MSA and 62 ± 6 years in UO-MSA, respectively. LO-MSA group included 4 MSA-C and 3 MSA-P, while UO-MSA group included 46 MSA-C and 30 MSA-P. LO-MSA included 5 men (71%) and 2 women (29%). UO-MSA included 44 men (58%) and 32 women (42%). All of the patients in the clinical cohort were Asian.
Table 1.
Demographic and clinical characteristics of cases in the clinical cohort
| Later onset | Usual Onset | P value | |
|---|---|---|---|
| Number of Patients | 7 | 76 | |
| Male, % (n) | 71 (5/7) | 58 (44/76) | 0.69 |
| Age at onset (years, mean ± SD) | 78.3 ± 2.8 | 62.0 ± 6.4 | <0.0001 |
| Initial symptoms, % (n) | |||
| Gait ataxia | 28.6 (2/7) | 42.1 (32/76) | 0.44 |
| Speech problem | 14.3 (1/7) | 11.8 (9/76) | > 0.99 |
| Hand clumsiness | 14.3 (1/7) | 10.5 (8/76) | 0.57 |
| Gait difficulty | 14.3 (1/7) | 9.2 (7/76) | 0.52 |
| Tremor | 0 (0/7) | 6.6 (5/76) | > 0.99 |
| Bradykinesia | 14.3 (1/7) | 3.9 (3/76) | 0.30 |
| falls | 0 (0/7) | 1.3 (1/76) | > 0.99 |
| Frequent urination | 14.3 (1/7) | 6.6 (5/76) | 0.42 |
| Urinary incontinence | 0 (0/7) | 2.6 (2/76) | > 0.99 |
| Urinary retention | 0 (0/7) | 1.3 (1/76) | > 0.99 |
| Orthostatic hypotension | 0 (0/7) | 2.6 (2/76) | > 0.99 |
| Pain | 0 (0/7) | 1.3 (1/76) | > 0.99 |
| MSA-P, % (n) | 42.9 (3/7) | 39.5 (30/76) | > 0.99 |
| MRI findings at the time of diagnosis, % (n) | |||
| Hot cross bun sign | 42.9 (3/7) | 53.9 (41/76) | 0.70 |
| Putaminal rim sign | 42.9 (3/7) | 18.4 (14/76) | 0.15 |
| Atrophy of putamen | 57.1 (4/7) | 46.1 (35/76) | 0.70 |
| Atrophy of pons | 57.1 (4/7) | 71.1 (54/76) | 0.43 |
| Atrophy of MCP | 14.3 (1/7) | 36/76 (47.4) | 0.12 |
| Atrophy of cerebellum | 71.4 (5/7) | 80.3 (61/76) | 0.63 |
| Symptoms at the time of diagnosis, % (n) | |||
| Cerebellar ataxia | 71.4 (5/7) | 89.5 (68/76) | 0.20 |
| Extrapyramidal symptoms | 71.4 (5/7) | 73.7 (56/76) | > 0.99 |
| Pyramidal signs | 71.4 (5/7) | 68.4 (52/76) | > 0.99 |
| Orthostatic hypotension | 57.1 (4/7) | 73.7 (56/76) | 0.39 |
| Urinary problems | 85.7 (6/7) | 85.5 (65/76) | > 0.99 |
| Cognitive impairment | 0 (0/7) | 1.3 (1/76) | > 0.99 |
| Downgaze palsy | 14.3 (1/7) | 1.3 (1/76) | 0.16 |
MSA, multiple system atrophy; MRI, magnetic resonance imaging; MCP, middle cerebellar peduncle
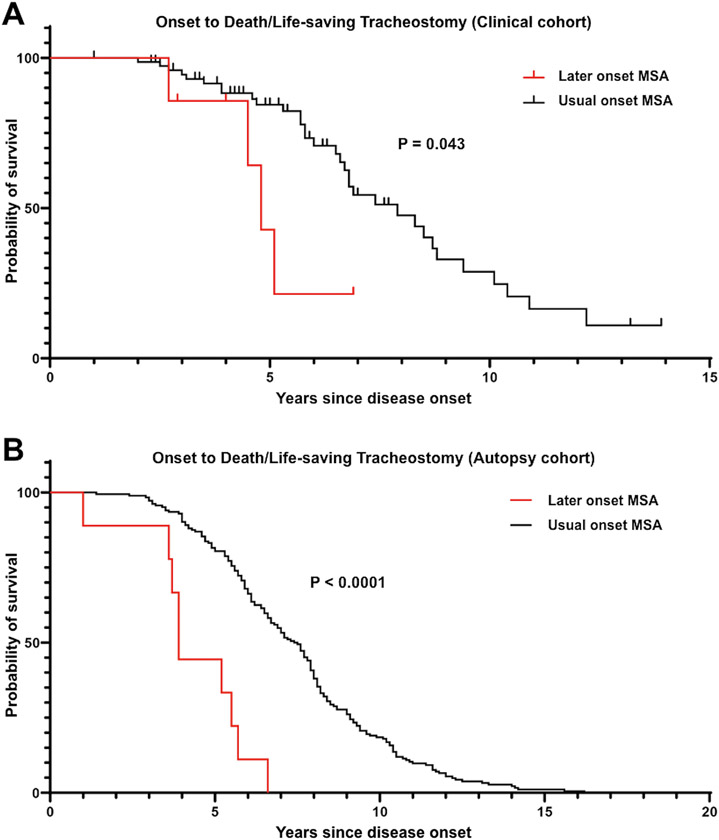
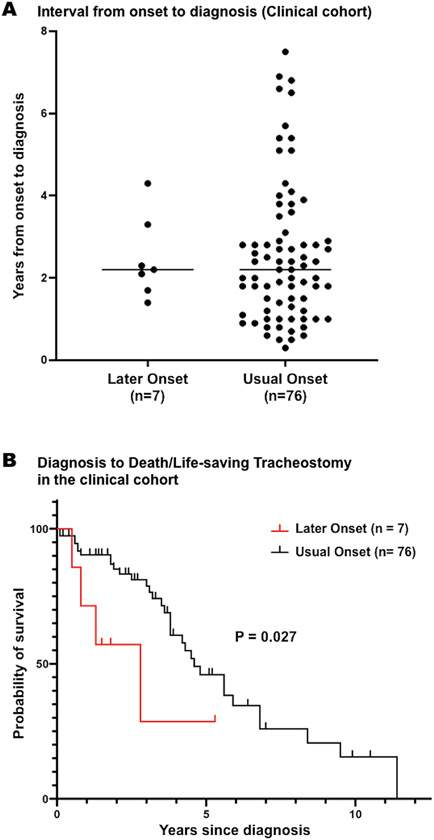
In general, the prognosis of MSA is poor. To examine whether LO-MSA patients have a worse prognosis, we compared survival in LO-MSA with UO-MSA. One of the common causes of death in MSA is respiratory failure due to pneumonia, asphyxiation, or vocal cord paralysis. In these life-threatening situations, without a tracheostomy, death would occur. Therefore, we compared the prognosis of the two groups using not only time to death but also time to tracheostomy in a life-threatening situation. None of LO-MSA patients and 22% of UO-MSA patients (17/76) underwent life-saving tracheostomy during the observation period. The median interval from the onset of illness to death or tracheostomy in a life-threatening situation was significantly shorter in LO-MSA than in UO-MSA (4.8 vs. 7.9 years; P = 0.043, Figure 2A). We examined the time from onset to diagnosis in each group. The interval from onset to diagnosis was not significantly different between LO-MSA and UO-MSA (2.5 ± 1.0 vs. 2.5 ± 1.7 years; P = 0.77, Figure 3A). On the contrary, the median duration from diagnosis to death or life-saving tracheostomy was significantly shorter in LO-MSA than in UO-MSA (2.8 years in LO-MSA vs. 4.6 years in UO-MSA; P = 0.03, Figure 3B).
Figure 2. Kaplan-Meier survival analysis of time from onset to death/life-saving tracheostomy.
(A) In the clinical cohort, LO-MSA patients showed significantly shorter survival compared with UO-MSA patients (P = 0.043).
(B) In the autopsy cohort, LO-MSA patients showed significantly shorter survival compared with UO-MSA patients (P < 0.0001).
Figure 3. Interval from onset to diagnosis and Kaplan-Meier survival analysis of survival from diagnosis to death/life-saving tracheostomy in the clinical cohort.
(A) Interval from onset to diagnosis was not significantly different between LO-MSA patients and UO-MSA patients.
(B) LO-MSA patients showed significantly shorter survival from diagnosis to death/life-saving tracheostomy in the clinical cohort (P = 0.027).
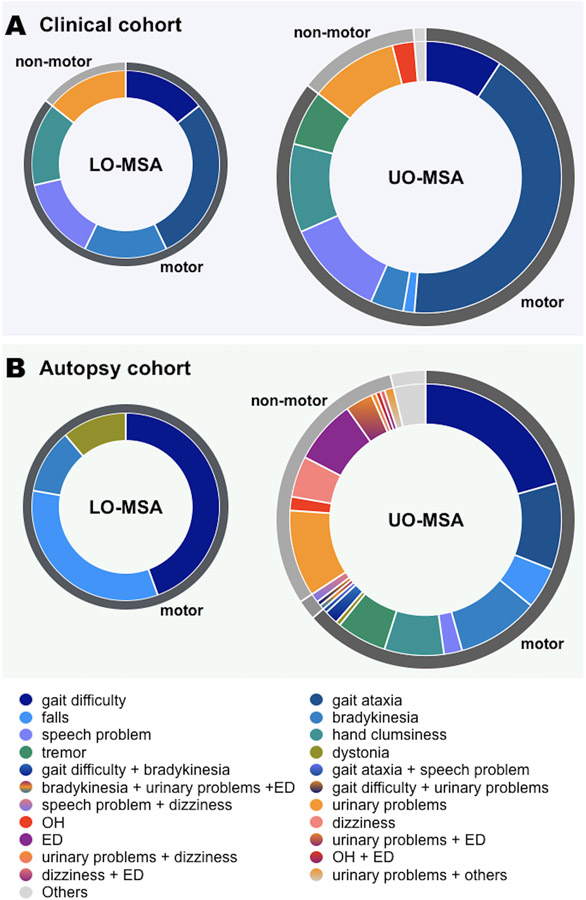
To understand the characteristics of LO-MSA patients, we investigated the initial symptoms in each group. Autonomic dysfunction was an initial symptom in 14% (1/7) in LO-MSA and 13% (10/76) in UO-MSA. The details of the initial symptoms in each group were summarized in Figure 4A. All patients underwent brain MRI at the time of diagnosis, and there was no significant difference in the frequency of MRI findings suggestive of MSA between LO-MSA and UO-MSA (Table 1). No patients showed a hummingbird sign, which is typical of progressive supranuclear palsy. Detailed MRI findings of each patient were summarized in Online resource 1. At the time of diagnosis, there was also no significant difference between the two groups in the presence of each clinical sign or symptom (Table 1). We provide detailed clinical signs and symptoms of each patient at the time of diagnosis in Online resource 2.
Figure 4. Initial symptoms in later onset MSA patients and usual onset MSA patients in each cohort.
(A) In the clinical cohort, the fraction of patients who presented with autonomic dysfunction as initial symptoms were 14% in LO-MSA (1/7) and 13% in UO-MSA (10/76).
(B) In the autopsy cohort, none of the LO-MSA patients had autonomic dysfunction as their initial symptom. In UO-MSA, the fractions of patients whose initial symptoms were autonomic dysfunction, motor symptoms, both simultaneously were 30% (56/184), 64% (117/184), and 2% (4/184), respectively.
OH, orthostatic hypotension; ED, erectile dysfunction
Autopsy cohort
Next, we collected the clinical information on MSA patients in the Mayo Clinic brain bank to validate the results in the clinical cohort. We identified 9 LO-MSA and 184 UO-MSA in the autopsy cohort. The frequency of LO-MSA was 5%. We summarize the characteristics of each group in Table 2. The mean age of onset was 81 ± 5 years in LO-MSA and 58 ± 8 years in UO-MSA. LO-MSA included 1 MSA-OPCA (11%) and 8 MSA-SND (89%). There was no “minimal change” MSA in LO-MSA. UO-MSA included of 38 MSA-OPCA (21%), 86 MSA-SND (47%), 57 MSA-SND/OPCA (31%), and 3 “minimal change” MSA (2%). No significant difference was found in pathological subtypes between LO-MSA and UO-MSA. LO-MSA included 3 men (37.5%) and 5 women (62.5%). UO-MSA included 97 men (53%) and 87 women (47%). Ninety-five percent of patients (183/193) were Caucasian.
Table 2.
Demographic and clinical characteristics of cases in the autopsy cohort
| Later onset | Usual Onset | P value | |
|---|---|---|---|
| Number of Patients | 9 | 184 | |
| Male, % (n) | 44.4 (4/9) | 52.7 (97/184) | 0.74 |
| Age at onset (years, mean ± SD) | 80.8 ± 4.5 | 57.7 ± 7.7 | < 0.0001 |
| Initial symptoms, % (n) | |||
| Gait difficulty | 44.4 (4/9) | 22.8 (42/184) | 0.22 |
| Bradykinesia | 11.1 (1/9) | 12.0 (22/184) | > 0.99 |
| Hand clumsiness | 0 (0/9) | 7.1 (13/184) | > 0.99 |
| Tremor | 0 (0/9) | 6.0 (11/184) | > 0.99 |
| Falls | 33.3 (3/9) | 4.9 (9/184) | 0.013 |
| Gait ataxia | 0 (0/9) | 10.3 (19/184) | 0.60 |
| Speech problem | 0 (0/9) | 3.8 (7/184) | > 0.99 |
| Dystonia | 11.1 (1/9) | 0.5 (1/184) | 0.091 |
| Apraxia | 0 (0/9) | 0.5 (1/184) | > 0.99 |
| Cramps | 0 (0/9) | 0.5 (1/184) | > 0.99 |
| Urinary incontinence | 0 (0/9) | 11.4 (21/184) | 0.60 |
| Urinary retention | 0 (0/9) | 4.3 (8/184) | > 0.99 |
| Frequent urination | 0 (0/9) | 1.1 (2/184) | > 0.99 |
| Orthostatic hypotension | 0 (0/9) | 2.2 (4/184) | > 0.99 |
| Dizziness | 0 (0/9) | 6.5 (12/184) | > 0.99 |
| Erectile dysfunction | 0 (0/4) | 23.7 (23/97) | 0.57 |
| Cognitive impairment | 0 (0/9) | 1.6 (3/184) | > 0.99 |
| Paraesthesia | 0 (0/9) | 1.1 (2/184) | > 0.99 |
| Stridor | 0 (0/9) | 0.5 (1/184) | > 0.99 |
| Pathological subtypes | |||
| MSA-SND | 88.9 (8/9) | 46.7 (86/184) | 0.065 |
| MSA-OPCA | 11.1 (1/9) | 20.7 (38/184) | > 0.99 |
| MSA-SND/OPCA | 0 (0/9) | 31.0 (57/184) | 0.24 |
| Minimal change MSA | 0 (0/9) | 1.6 (3/184) | > 0.99 |
| Pathological features | |||
| Brain weight (g) | 1143 ± 150 | 1222 ± 144 | 0.11 |
| Braak NFT stage | 2.4 ± 1.6 | 1.5 ± 1.1 | 0.017 |
| Thal amyloid phase | 2.0 ± 1.4 | 0.9 ± 1.3 | 0.017 |
| Presence of vascular pathology, % (n) | 11.1 (1/9) | 10.3 (19/184) | > 0.99 |
MSA, multiple system atrophy; SND, striatonigral degeneration; OPCA, olivopontocerebellar atrophy; NFT, neurofibrillary tangle
We found that the prognosis of LO-MSA patients was worse than that of UO-MSA in the clinical cohort. To validate this result, we compared the time between onset and death or life-saving tracheostomy in the autopsy cohort. None of LO-MSA patients and 4% of UO-MSA patients (7/193) experienced life-saving tracheostomy. The median interval from the onset of illness to death or tracheostomy in a life-threatening situation was significantly shorter in LO-MSA than in UO-MSA (3.9 vs. 7.5 years; P < 0.0001, Figure 2B), which was consistent with the result in the clinical cohort.
All LO-MSA patients presented with motor symptoms as their initial symptoms (4 with gait difficulty, 3 with falls, 1 with bradykinesia, and 1 with dystonia). In UO-MSA, 118 patients (64%) presented with motor symptoms, 55 patients (30%) presented with autonomic dysfunction, 6 patients (3%) presented with other symptoms (2 with cognitive impairment, 2 with paraesthesia, 1 with dystonia, and 1 with stridor), 4 patients (2%) had motor symptoms and autonomic dysfunction simultaneously at the presentation (2 with dysarthria and dizziness, 1 with bradykinesia, erectile dysfunction, and urinary incontinence, and 1 with gait difficulty and frequent urination), and 1 patient presented with urinary incontinence and cognitive impairment. Although not significantly different, UO-MSA patients tended to have more autonomic dysfunction as an initial symptom than LO-MSA patients (P = 0.059, Figure 4B).
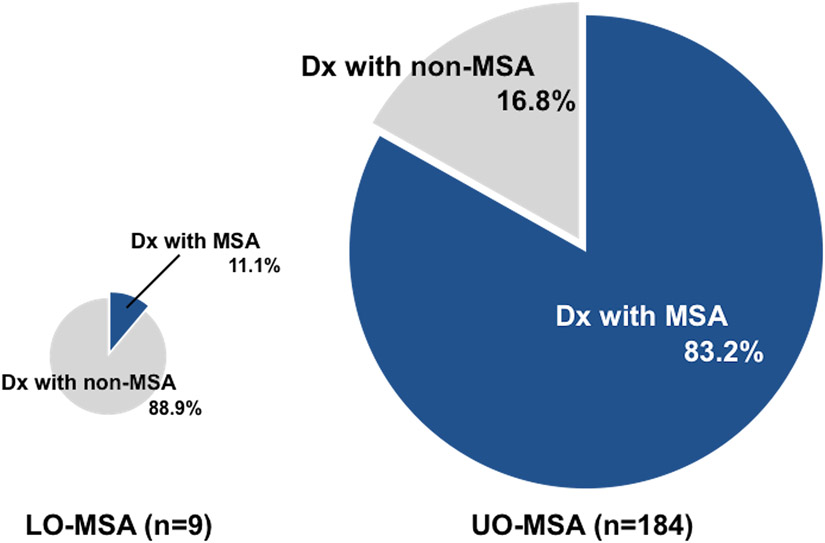
Approximately 20% of MSA patients in the autopsy cohort were not given an antemortem diagnosis of MSA. Among the patients who were not diagnosed with MSA before death (including those in which MSA was included in the differential diagnosis), progressive supranuclear palsy was the most common, followed by Parkinson’s disease, dementia with Lewy bodies, and corticobasal degeneration. The rate of confirmed diagnosis of MSA before death was 83% (153/184) in UO-MSA, but only 11% (1/9) in LO-MSA. The frequency of antemortem diagnosis of MSA was significantly lower in LO-MSA than in UO-MSA (P < 0.0001, Figure 5). Among the pathological subtypes, the diagnostic accuracy was 71% in MSA-SND (61/86), 91% in MSA-OPCA (32/35), 87% in MSA-mixed (60/69). The diagnostic accuracy in MSA-SND was significantly lower than in other subtypes (P = 0.0086).
Figure 5. Antemortem diagnosis accuracy in the autopsy cohort.
In the autopsy cohort, 83% of UO-MSA patients were diagnosed with MSA before death, whereas only 11% of LO-MSA patients were diagnosed with MSA before death.
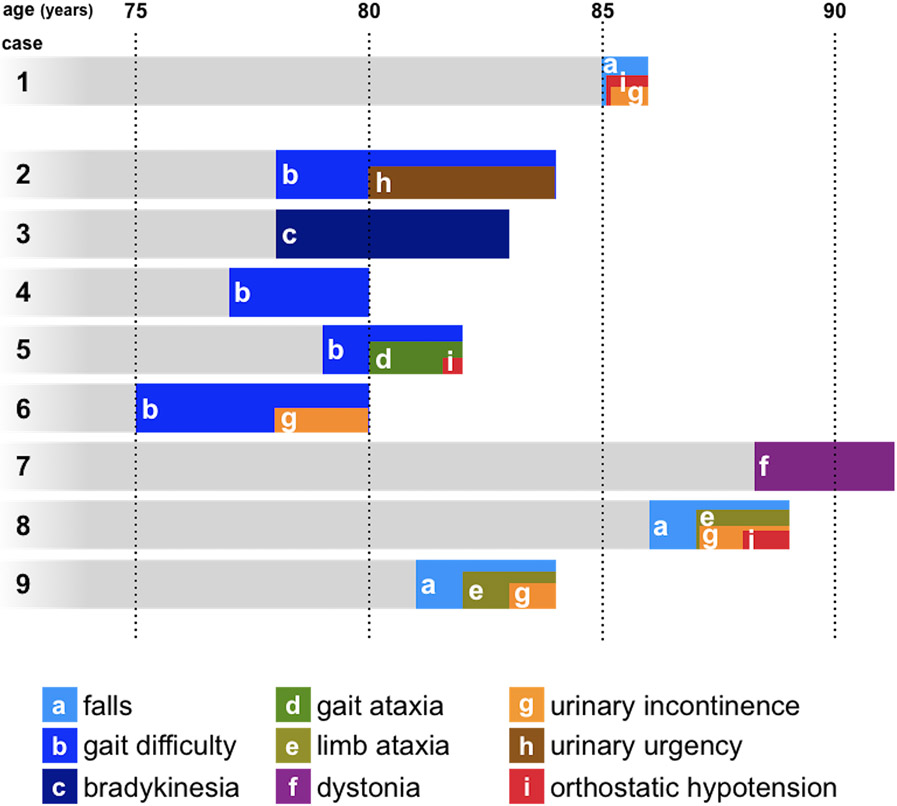
To further examine the reason for this low diagnostic accuracy in LO-MSA, we investigated the clinical course of LO-MSA in the autopsy cohort. We summarized clinical symptoms in LO-MSA patients in Table 3 and clinical courses in Figure 6. During the whole course, 2 patients developed slight truncal ataxia (case 5 and case 8) and 1 patient showed bilateral limb ataxia (Case 9). Urinary incontinence was found in 4 cases, two of which were considered due to slow movement speed. Orthostatic hypotension was found in 3 cases and leads to a diagnosis of MSA in one patient with syncope (case 1). In case 9, MSA was suspected in the clinical course, but the attending neurologist documented “her age certainly goes against that diagnosis” and could not confirm the diagnosis of MSA.
Table 3.
Clinical diagnosis and symptoms of LO-MSA patients in the autopsy cohort
| Sex | Age at onset |
Age at death |
Initial diagnosis |
Final diagnosis |
Reasons for diagnosis |
Initial symptoms |
Ataxia | Urinary problems | Orthostatic hypotension | Probable or possible, retrospectively |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Description | Age | Description | Age | Description | |||||||||
| 1 | M | 85 | 86 | MSA | MSA | Developing severe OH soon after falls | Falls | - | - | 85 | Urinary incontinence | 85 | sBP drop > 30 mmHg Dizziness and syncope | Probable |
| 2 | M | 78 | 84 | PSP vs MSA | PSP | Early falls | Gait difficulty | - | - | 80 | Urinary urgency | - | - | Possible |
| 3 | F | 78 | 83 | PSP | PSP | Early falls, neck rigidity, downgaze limitation | Bradykinesia | - | - | - | - | - | - | - |
| 4 | F | 77 | 80 | PSP vs MSA | PSP vs MSA | Early falls | Gait difficulty | - | - | - | - | - | - | - |
| 5 | M | 79 | 82 | PD | PD | Resting tremor | Gait difficulty | 80 | Slightly wide-based gait | - | - | 82 | sBP drop > 30 mmHg Dizziness, but no syncope | Probable |
| 6 | M | 75 | 80 | PSP | PSP | Neck rigidity, FoG, eyelid-opening apraxia | Gait difficulty | - | - | 78 | Urinary incontinence (considered due to slow mobility) | - | - | Probable |
| 7 | F | 88 | 91 | CBS | CBS | Left dominant dystonia and contracture | Dystonia | - | - | - | - | - | - | - |
| 8 | F | 86 | 89 | PSP | PSP | Early falls, downgaze palsy | Falls | 87 | Slightly wide-based gait | 87 | Urinary incontinence (considered due to slow mobility) | 88 | sBP drop > 30 mmHg Dizziness, but no syncope | Probable |
| 9 | F | 81 | 86 | PD vs PSP | MSA vs PSP | Early falls, age at onset | Falls | 84 | Bilateral dysmetria on finger-to-nose test | 85 | Urinary incontinence | - | - | Probable |
MSA, multiple system atrophy; OH, orthostatic hypotension; sBP, systolic blood pressure; PSP, progressive supranuclear palsy; PD, Parkinson’s disease; FoG, freezing of gait; CBS, corticobasal syndrome
Figure 6. Clinical courses of LO-MSA patients in the autopsy cohort.
The figure indicates the clinical course of how each LO-MSA patient developed each symptom after onset.
There was no significant difference in brain weight and presence of vascular pathology between LO-MSA and UO-MSA (P = 0.11 and P > 0.99), whereas the Braak NFT stage and Thal amyloid phase were significantly higher in LO-MSA than those in UO-MSA (P = 0.017 and P = 0.017). There was no significant correlation between disease duration and the Braak NFT stage or Thal amyloid phase in either LO-MSA (P = 0.72 and P = 0.46) or UO-MSA (P = 0.57 and P = 0.27).
Discussion
Here we report the frequency and clinical features of LO-MSA in a clinical cohort and compare these observations in autopsy-confirmed MSA. There are three major findings from this study. First, MSA can occur in individuals older than 75 years of age. Second, the prognosis is poor in patients with LO-MSA, and the time for decision-making is limited. Finally, clinical diagnosis of MSA may be difficult in elderly individuals, probably because of the paucity of autonomic symptoms as initial symptoms and the fact that late age of onset is considered a “non-supportive feature” in the current criteria.
Patients with LO-MSA accounted for 8% in the clinical cohort and 5% in the autopsy cohort. Although the nature of the two cohorts differed in terms of the ethnic composition and diagnostic certainty (i.e., clinical diagnosis vs. pathologic diagnosis), both cohorts had a similar proportion of LO-MSA. A previous study showed that LO-MSA accounted for 23% (128 UO-MSA and 39 LO-MSA) in the clinical cohort in Korea and 6% (155 UO-MSA and 10 LO-MSA) in the autopsy cohort in Japan [19]. Our results were compatible with the result in the autopsy cohort in the previous study. Therefore, although rare, clinicians may encounter older patients presenting with MSA in clinical practice, and this diagnosis should be kept in mind. The oldest age of symptomatic onset in the autopsy cohort was 88 years, indicating that MSA cannot be ruled out simply by age of onset.
A previous study reported poor prognosis in MSA patients with older age at onset [37], but another study on LO-MSA did not confirm this observation [19]. They analyzed a clinical cohort in Korea (128 UO-MSA and 39 LO-MSA) and an autopsy cohort in Japan (155 UO-MSA and 10 LO-MSA). They reported that the disease duration was significantly shorter in LO-MSA than that in UO-MSA in the autopsy cohort, but that the disease duration from onset to wheelchair-bound state was not significantly different in the clinical cohort. Therefore, we examined the prognosis of LO-MSA in the present study. As a result, we found that LO-MSA had a worse prognosis than UO-MSA in both clinical and autopsy cohorts. The median or mean survival time for MSA has been reported to be 6 to 10 years [1, 5, 8, 21, 24, 37, 39, 40]. In our cohorts, the median time from onset to death or life-saving tracheostomy was 7.5 years in UO-MSA, which is compatible with previous reports. On the other hand, the median time from onset to death was less than 5 years in LO-MSA. In the clinical cohort, the average age of onset of LO-MSA was 78 years. The life expectancy data for 2018 for 80-year-old individuals in Japan is 9 years in men and 12 years in women in Japan in 2018 [38]. Therefore, the poor prognosis in LO-MSA cannot merely be attributed to shortened life expectancy in this age, but rather to the disease, itself. In fact, the cause of death in all of the four LO-MSA patients who died during the observation period was respiratory failure with aspiration pneumonia. In addition to the poorer prognosis in LO-MSA in the clinical cohort, we found that the median duration from diagnosis to death or life-saving tracheostomy was shorter than 3 years in LO-MSA. The present results indicate that LO-MSA patients and their families have a limited time to accept the diagnosis, adjust to the disease condition, and make a decision about life-prolonging procedures, such as tracheostomy.
In the present study, in addition to death, we employed life-saving tracheostomy as an endpoint because death will occur unless a tracheostomy is performed in case of respiratory failure due to severe pneumonia, vocal cord paralysis, or asphyxia. In some of the deaths included in the study, a tracheostomy would have prolonged life. The time to death is partially affected by whether the patient undergoes a tracheostomy. A previous study indicated that tracheostomy prolonged survival in MSA [23]. Therefore, we thought it would be more realistic to consider life-saving tracheostomy in addition to time to death when comparing prognosis. In this study, there were no LO-MSA patients who underwent tracheostomy. Hence, when we exclude life-saving tracheostomy from the endpoint and consider only death as the endpoint, the time from onset to death would be longer only in UO-MSA. As a result, the prognosis of LO-MSA is even worse than UO-MSA when compared by time from onset to death.
Complicating the situation is the difficulty of making an accurate diagnosis in MSA in elderly patients. Among 9 LO-MSA patients in the autopsy cohort, 8 patients could not confirm the diagnosis of MSA before their death in the present study. There are several reasons for this. First, clinicians may hesitate to diagnose MSA when the patient is very old because the onset age at 75 or greater is one of the non-supporting features for MSA [9]. In fact, the attending neurologist documented that the old age at onset was against the diagnosis of MSA in one LO-MSA patient. Second, LO-MSA may begin with motor symptoms, such as gait disturbances and falls, that are multifactorial in the elderly, and less often with autonomic symptoms, which themselves are common in the elderly. When we examined the entire clinical course, 5 LO-MSA patients without confirmed MSA diagnosis showed either ataxia, urinary problems, or orthostatic hypotension (Table 3, Figure 6). In this way, many LO-MSA patients developed clinical symptoms suspicious of MSA, including subtle signs, during the course of the disease. The diagnostic accuracy of MSA may improve with inclusion of such symptoms. In the clinical cohort, 86% (6/7) of LO-MSA patients showed MRI abnormalities suggestive of MSA at the time of diagnosis, which indicates the usefulness of MRI in the diagnosis of MSA in elderly patients. Clinicians should have MSA in the differential diagnosis for older patients with extrapyramidal symptoms or cerebellar ataxia and consider ancillary tests to detect autonomic dysfunction and brain MRI for an accurate diagnosis.
The second consensus statement on MSA [9] has increased diagnostic sensitivity of MSA [25] and it has been widely used. The consensus criteria include several non-supporting features, such as pill-rolling tremor, significant neuropathy, family history of ataxia or Parkinsonism, dementia, and onset after age 75. Several of these non-supporting features have been criticized [32]. For example, familial MSA cases have been reported [10]. Cognitive impairment is more common in MSA than previously considered [12, 31]. In addition, there are increasing reports, including the present study, of LO-MSA [19, 28]. Therefore, although rare entity, the existence of MSA cases with “onset after age 75” should be considered.
A potential limitation of the current study is the possibility of incorrect diagnosis of MSA in the clinical cohort. At the time of diagnosis, each patient was admitted to hospitals that have physicians with expertise in intractable neurological diseases and a municipal or prefectural intractable disease center. There, they underwent medical evaluation by multiple board-certificated neurologists and were confirmed to fulfill the diagnostic criteria. All patients underwent MRI and no patients showed a humming-bird sign suggestive of PSP, whereas most patients presented abnormalities suggestive of MSA such as hot cross bun sign, hyperintense putaminal rim, putaminal atrophy, pontine atrophy, or cerebellar atrophy. In addition, we could confirm similar results about the frequency and the poorer prognosis of LO-MSA in the autopsy cohort. Nevertheless, we could not describe how many possible MSA patients progressed in probable MSA in the follow-up because detailed records on the evaluation on orthostatic hypotension or urodynamic studies were unavailable in the outpatient clinic. This may lower the diagnostic accuracy.
Another limitation is the possibility of overestimation of the poor prognosis of LO-MSA in the autopsy cohort. The cause of death of LO-MSA patients in the autopsy cohort was not documented, except for one case of aspiration pneumonia. It remains possible that the LO-MSA patients died early due to other complications. On the other hand, all deaths of LO-MSA patients who died during the observation period in the clinical cohort were attributed to MSA progression. Therefore, we considered LO-MSA to have a worse prognosis than UO-MSA.
The other limitation of the present study lies in the inherent nature of the retrospective design. The data from the present study should be interpreted with caution. The prevalence of MSA is between 1.9 and 4.9 cases per 100,000 population [2, 29, 34]. Given the frequency from our findings, the incidence of LO-MSA can be estimated to be 0.1 - 0.25 per 100,000. Prospective studies of a rare presentation of a rare disorder, such as LO-MSA, are impractical. Nevertheless, the present study provides insight into the differential diagnosis of autonomic, gait, and cerebellar disorders in the elderly.
Conclusion
The present study indicates that LO-MSA accounts for about 6% of MSA. Thus, although rare, MSA should be considered even with onset after age 75. The prognosis of LO-MSA is worse than UO-MSA, and it is important to be aware of the limited time available after diagnosis to decide on a treatment plan.
Supplementary Material
Acknowledgements
We would like to express our sincere gratitude to patients and their family members for their agreement to brain donation. We also appreciate the dedicated work of members in incurable disease canters.
Funding
This study is supported by NIH grant (NS110435) and the Rainwater Charitable Foundation.
Footnotes
Conflicts of interest
The authors declare no financial or other conflicts of interest.
Ethics approval
All brain autopsies were performed with the consent of the legal next-of-kin or an individual with legal authority to grant permission for autopsy. Deidentified studies using these autopsy samples are considered exempt from human subject research by the Mayo Clinic Institutional Review Board. The study on the clinical cohort was approved by Kobe University Ethical Committee.
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
- 1.Ben-Shlomo Y, Wenning GK, Tison F, Quinn NP (1997) Survival of patients with pathologically proven multiple system atrophy: a meta-analysis. Neurology 48:384–393 [DOI] [PubMed] [Google Scholar]
- 2.Bower JH, Maraganore DM, McDonnell SK, Rocca WA (1997) Incidence of progressive supranuclear palsy and multiple system atrophy in Olmsted County, Minnesota, 1976 to 1990. Neurology 49:1284–1288 [DOI] [PubMed] [Google Scholar]
- 3.Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82:239–259 [DOI] [PubMed] [Google Scholar]
- 4.Chelban V, Bocchetta M, Hassanein S, Haridy NA, Houlden H, Rohrer JD (2019) An update on advances in magnetic resonance imaging of multiple system atrophy. J Neurol 266:1036–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coon EA, Sletten DM, Suarez MD, Mandrekar JN, Ahlskog JE, Bower JH, Matsumoto JY, Silber MH, Benarroch EE, Fealey RD, Sandroni P, Low PA, Singer W (2015) Clinical features and autonomic testing predict survival in multiple system atrophy. Brain 138:3623–3631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cykowski MD, Coon EA, Powell SZ, Jenkins SM, Benarroch EE, Low PA, Schmeichel AM, Parisi JE (2015) Expanding the spectrum of neuronal pathology in multiple system atrophy. Brain 138:2293–2309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fanciulli A, Wenning GK (2015) Multiple-system atrophy. N Engl J Med 372:249–263 [DOI] [PubMed] [Google Scholar]
- 8.Figueroa JJ, Singer W, Parsaik A, Benarroch EE, Ahlskog JE, Fealey RD, Parisi JE, Sandroni P, Mandrekar J, Iodice V, Low PA, Bower JH (2014) Multiple system atrophy: prognostic indicators of survival. Mov Disord 29:1151–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, Wood NW, Colosimo C, Dürr A, Fowler CJ, Kaufmann H, Klockgether T, Lees A, Poewe W, Quinn N, Revesz T, Robertson D, Sandroni P, Seppi K, Vidailhet M (2008) Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71:670–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hara K, Momose Y, Tokiguchi S, Shimohata M, Terajima K, Onodera O, Kakita A, Yamada M, Takahashi H, Hirasawa M, Mizuno Y, Ogata K, Goto J, Kanazawa I, Nishizawa M, Tsuji S (2007) Multiplex families with multiple system atrophy. Arch Neurol 64:545–551 [DOI] [PubMed] [Google Scholar]
- 11.Kim HJ, Jeon BS, Jellinger KA (2015) Diagnosis and differential diagnosis of MSA: boundary issues. J Neurol 262:1801–1813 [DOI] [PubMed] [Google Scholar]
- 12.Kitayama M, Wada-Isoe K, Irizawa Y, Nakashima K (2009) Assessment of dementia in patients with multiple system atrophy. Eur J Neurol 16:589–594 [DOI] [PubMed] [Google Scholar]
- 13.Koga S, Aoki N, Uitti RJ, van Gerpen JA, Cheshire WP, Josephs KA, Wszolek ZK, Langston JW, Dickson DW (2015) When DLB, PD, and PSP masquerade as MSA: an autopsy study of 134 patients. Neurology 85:404–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koga S, Cheshire WP, Tipton PW, Driver-Dunckley ED, Wszolek ZK, Uitti RJ, Graff-Radford NR, van Gerpen JA, Dickson DW (2021) Clinical features of autopsy-confirmed multiple system atrophy in the Mayo Clinic Florida brain bank. Parkinsonism Relat Disord 89:155–161 [DOI] [PubMed] [Google Scholar]
- 15.Koga S, Dickson DW (2019) "Minimal change" multiple system atrophy with limbic-predominant α-synuclein pathology. Acta Neuropathol 137:167–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koga S, Dickson DW (2018) Recent advances in neuropathology, biomarkers and therapeutic approach of multiple system atrophy. J Neurol Neurosurg Psychiatry 89:175–184 [DOI] [PubMed] [Google Scholar]
- 17.Kon T, Mori F, Tanji K, Miki Y, Wakabayashi K (2013) An autopsy case of preclinical multiple system atrophy (MSA-C). Neuropathology 33:667–672 [DOI] [PubMed] [Google Scholar]
- 18.Laurens B, Vergnet S, Lopez MC, Foubert-Samier A, Tison F, Fernagut PO, Meissner WG (2017) Multiple System Atrophy - State of the Art. Curr Neurol Neurosci Rep 17:41. [DOI] [PubMed] [Google Scholar]
- 19.Lee YH, Ando T, Lee JJ, Baek MS, Lyoo CH, Kim SJ, Kim M, Cho JW, Sohn YH, Katsuno M, Watanabe H, Yoshida M, Lee PH (2020) Later-Onset Multiple System Atrophy: A Multicenter Asian Study. Mov Disord 35:1692–1693 [DOI] [PubMed] [Google Scholar]
- 20.Ling H, Asi YT, Petrovic IN, Ahmed Z, Prashanth LK, Hazrati LN, Nishizawa M, Ozawa T, Lang A, Lees AJ, Revesz T, Holton JL (2015) Minimal change multiple system atrophy: an aggressive variant? Mov Disord 30:960–967 [DOI] [PubMed] [Google Scholar]
- 21.Low PA, Reich SG, Jankovic J, Shults CW, Stern MB, Novak P, Tanner CM, Gilman S, Marshall FJ, Wooten F, Racette B, Chelimsky T, Singer W, Sletten DM, Sandroni P, Mandrekar J (2015) Natural history of multiple system atrophy in the USA: a prospective cohort study. Lancet Neurol 14:710–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakazato Y, Yamazaki H, Hirato J, Ishida Y, Yamaguchi H (1990) Oligodendroglial microtubular tangles in olivopontocerebellar atrophy. J Neuropathol Exp Neurol 49:521–530 [DOI] [PubMed] [Google Scholar]
- 23.Nishida K, Sakashita K, Yamasaki H, Futamura N (2022) Impact of tracheostomy invasive ventilation on survival in Japanese patients with multiple system atrophy. Parkinsonism Relat Disord [DOI] [PubMed] [Google Scholar]
- 24.O'Sullivan SS, Massey LA, Williams DR, Silveira-Moriyama L, Kempster PA, Holton JL, Revesz T, Lees AJ (2008) Clinical outcomes of progressive supranuclear palsy and multiple system atrophy. Brain 131:1362–1372 [DOI] [PubMed] [Google Scholar]
- 25.Osaki Y, Ben-Shlomo Y, Lees AJ, Wenning GK, Quinn NP (2009) A validation exercise on the new consensus criteria for multiple system atrophy. Mov Disord 24:2272–2276 [DOI] [PubMed] [Google Scholar]
- 26.Palma JA, Norcliffe-Kaufmann L, Kaufmann H (2018) Diagnosis of multiple system atrophy. Auton Neurosci 211:15–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papp MI, Kahn JE, Lantos PL (1989) Glial cytoplasmic inclusions in the CNS of patients with multiple system atrophy (striatonigral degeneration, olivopontocerebellar atrophy and Shy-Drager syndrome). J Neurol Sci 94:79–100 [DOI] [PubMed] [Google Scholar]
- 28.Sakushima K, Nishimoto N, Nojima M, Matsushima M, Yabe I, Sato N, Mori M, Sasaki H (2015) Epidemiology of Multiple System Atrophy in Hokkaido, the Northernmost Island of Japan. Cerebellum 14:682–687 [DOI] [PubMed] [Google Scholar]
- 29.Schrag A, Ben-Shlomo Y, Quinn NP (1999) Prevalence of progressive supranuclear palsy and multiple system atrophy: a cross-sectional study. Lancet 354:1771–1775 [DOI] [PubMed] [Google Scholar]
- 30.Sekiya H, Kowa H, Koga H, Takata M, Satake W, Futamura N, Funakawa I, Jinnai K, Takahashi M, Kondo T, Ueno Y, Kanagawa M, Kobayashi K, Toda T (2019) Wide distribution of alpha-synuclein oligomers in multiple system atrophy brain detected by proximity ligation. Acta Neuropathol 137:455–466 [DOI] [PubMed] [Google Scholar]
- 31.Stankovic I, Krismer F, Jesic A, Antonini A, Benke T, Brown RG, Burn DJ, Holton JL, Kaufmann H, Kostic VS, Ling H, Meissner WG, Poewe W, Semnic M, Seppi K, Takeda A, Weintraub D, Wenning GK (2014) Cognitive impairment in multiple system atrophy: a position statement by the Neuropsychology Task Force of the MDS Multiple System Atrophy (MODIMSA) study group. Mov Disord 29:857–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stankovic I, Quinn N, Vignatelli L, Antonini A, Berg D, Coon E, Cortelli P, Fanciulli A, Ferreira JJ, Freeman R, Halliday G, Höglinger GU, Iodice V, Kaufmann H, Klockgether T, Kostic V, Krismer F, Lang A, Levin J, Low P, Mathias C, Meissner WG, Kaufmann LN, Palma JA, Panicker JN, Pellecchia MT, Sakakibara R, Schmahmann J, Scholz SW, Singer W, Stamelou M, Tolosa E, Tsuji S, Seppi K, Poewe W, Wenning GK (2019) A critique of the second consensus criteria for multiple system atrophy. Mov Disord 34:975–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thal DR, Rüb U, Orantes M, Braak H (2002) Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology 58:1791–1800 [DOI] [PubMed] [Google Scholar]
- 34.Tison F, Yekhlef F, Chrysostome V, Sourgen C (2000) Prevalence of multiple system atrophy. Lancet 355:495–496 [DOI] [PubMed] [Google Scholar]
- 35.Trojanowski JQ, Revesz T (2007) Proposed neuropathological criteria for the post mortem diagnosis of multiple system atrophy. Neuropathol Appl Neurobiol 33:615–620 [DOI] [PubMed] [Google Scholar]
- 36.Wakabayashi K, Mori F, Nishie M, Oyama Y, Kurihara A, Yoshimoto M, Kuroda N (2005) An autopsy case of early ("minimal change") olivopontocerebellar atrophy (multiple system atrophy-cerebellar). Acta Neuropathol 110:185–190 [DOI] [PubMed] [Google Scholar]
- 37.Watanabe H, Saito Y, Terao S, Ando T, Kachi T, Mukai E, Aiba I, Abe Y, Tamakoshi A, Doyu M, Hirayama M, Sobue G (2002) Progression and prognosis in multiple system atrophy: an analysis of 230 Japanese patients. Brain 125:1070–1083 [DOI] [PubMed] [Google Scholar]
- 38.Welfare MoHLa (2016) Summary of Simplified Life Tables, 2016. In:
- 39.Wenning GK, Ben Shlomo Y, Magalhães M, Daniel SE, Quinn NP (1994) Clinical features and natural history of multiple system atrophy. An analysis of 100 cases. Brain 117 ( Pt 4):835–845 [DOI] [PubMed] [Google Scholar]
- 40.Wenning GK, Geser F, Krismer F, Seppi K, Duerr S, Boesch S, Köllensperger M, Goebel G, Pfeiffer KP, Barone P, Pellecchia MT, Quinn NP, Koukouni V, Fowler CJ, Schrag A, Mathias CJ, Giladi N, Gurevich T, Dupont E, Ostergaard K, Nilsson CF, Widner H, Oertel W, Eggert KM, Albanese A, del Sorbo F, Tolosa E, Cardozo A, Deuschl G, Hellriegel H, Klockgether T, Dodel R, Sampaio C, Coelho M, Djaldetti R, Melamed E, Gasser T, Kamm C, Meco G, Colosimo C, Rascol O, Meissner WG, Tison F, Poewe W (2013) The natural history of multiple system atrophy: a prospective European cohort study. Lancet Neurol 12:264–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wenning GK, Quinn N, Magalhăes M, Mathias C, Daniel SE (1994) "Minimal change" multiple system atrophy. Mov Disord 9:161–166 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.