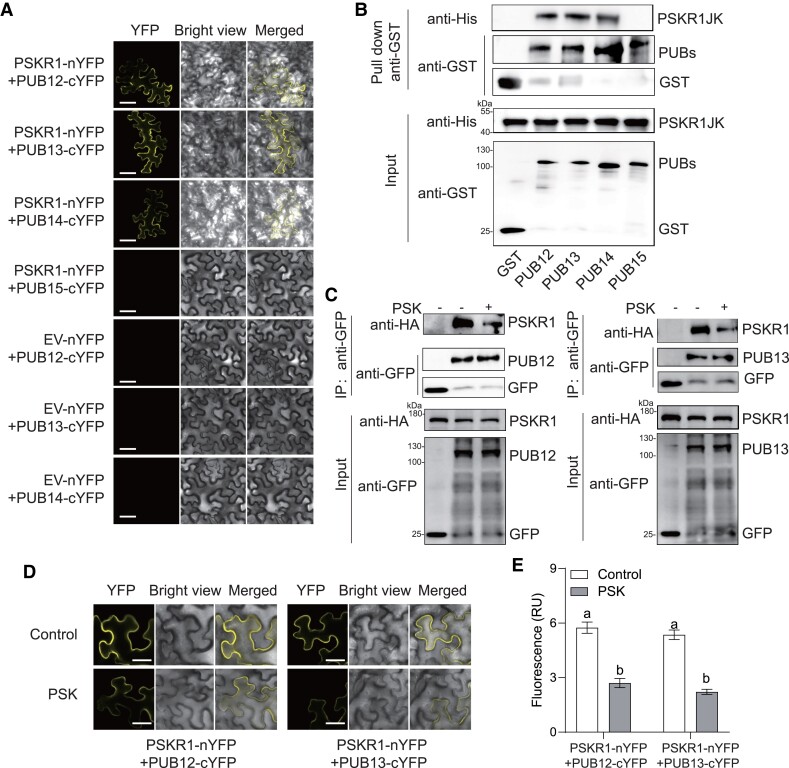
Figure 3.
PSK induced the dissociation of PSKR1 from PUB12/13. A) BiFC analyses of the binding between PSKR1 and PUBs. Agrobacterium carrying the indicated P2YN/C constructs were inoculated to 5-wk-old fully expanded N. benthamiana leaves. YFP fluorescence was visualized by confocal microscopy at 48 h after inoculation. Bar = 50 μm. B) PSKR1 interacted with PUB12/13/14 in an in vitro GST-pull-down assay. His-fusion PSKR1JK protein was incubated with glutathione beads coupled with GST-PUB12, GST-PUB13, GST-PUB14, GST-PUB15, and GST control for 3 h. Then, the beads were collected and washed for anti-His and anti-GST immunoblots. The input fusion proteins were indicated by immunoblots with anti-His antibody and anti-GST antibody, respectively. C) Co-IP analyses of the associations between HA-tagged PSKR1 and GFP-tagged PUB12/13 with or without application of 1 μM PSK. Five-wk-old fully expanded N. benthamiana leaves were contransfected with PSKR1-HA and PUB12-GFP, PUB13-GFP, or GFP control. After 48 h of inoculation, half of the leaves were infiltrated with 1 μM PSK, and the other parts were infiltrated with dH2O control for 1 h before the sample collection for Co-IP. The associations were detected by anti-HA and anti-GFP immunoblots. The input proteins before IP were detected by anti-HA immunoblot and anti-GFP antibody, respectively. D) Changes in the BiFC fluorescence signal of PSKR1-PUB12 and PSKR1-PUB13 interaction with or without application of 1 μM PSK for 2 h. Bar = 25 μm. E) The fluorescence signal intensity from (D) from three independent repeats was quantified and the data are shown as means of three biological replicates (± SD, n = 3), and different letters indicate significant differences (P < 0.05) according to Tukey's test.