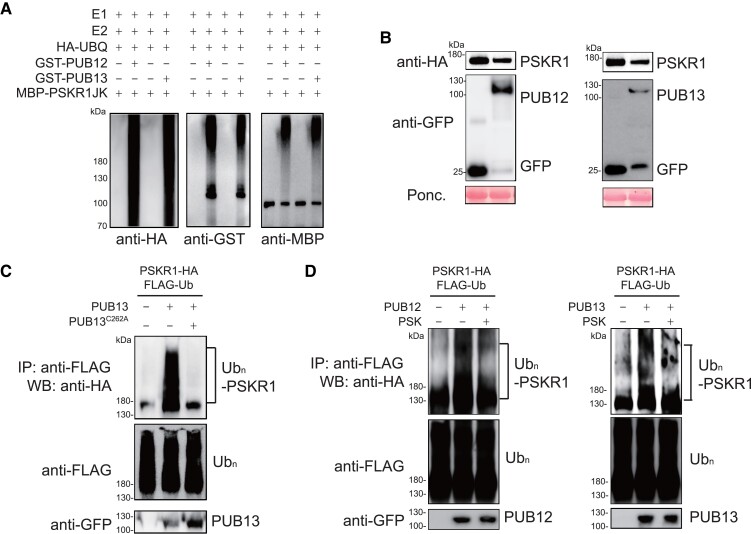
Figure 5.
PSK inhibited PUB12/13-meidated PSKR1 ubiquitination. A) PUB12/13 ubiquitinates PSKR1 in vitro. Ubiquitination of MBP-PSKR1JK was analyzed in the presence of E1, E2, ubiquitin, and GST-PUB12 or GST-PUB13 by immunoblots with an anti-HA antibody and anti-GST antibody, respectively. B) PUB12/13 reduced PSKR1 protein abundance. Agrobacterium carrying the indicated binary vectors were inoculated to 5-wk-old fully expanded N. benthamiana leaves. After 2-d of inoculation, samples were collected for immunoblots with anti-HA antibody and anti-GFP antibody, respectively. Ponceau S (Ponc.) staining was used as a protein loading control. C) PUB13 ubiquitinates PSKR1 in vivo. Five-wk-old fully expanded N. benthamiana leaves were cotransfected with FLAG-tagged ubiquitin (FLAG-Ub), HA-tagged PSKR1, and together with GFP-tagged PUB13, PUB13C262A (blocking E2-binding mutant), or a control GFP vector. After 2 d of transient expression, the samples were treated with 2 μM MG132 for 3 h and collected. The ubiquitinated PSKR1 was detected with an anti-HA WB after anti-FLAG immunoprecipitation (top). The total ubiquitinated proteins were evaluated by an anti-HA WB (middle) and the input PUB13 proteins were detected by an anti-GFP WB (Bottom). D) PSK suppressed PUB12/13-mediated ubiquitination of PSKR1 in planta. Five-wk-old N. benthamiana leaves were cotransfected with FLAG-Ub, PSKR1-HA, and together with PUB12-GFP, PUB13-GFP, or GFP control. After 2 d of transient expression, the leaves were further infiltrated with 1 μM PSK for 3 h in the presence of 2 μM MG132 before sample collection. The ubiquitinated PSKR1 was detected by an anti-HA antibody after immunoprecipitation with an anti-FLAG antibody (top). The total ubiquitinated proteins (middle) and input GFP-tagged PUB proteins (bottom) were indicated with the immunoblot with an anti-FLAG antibody and an anti-GFP antibody, respectively.