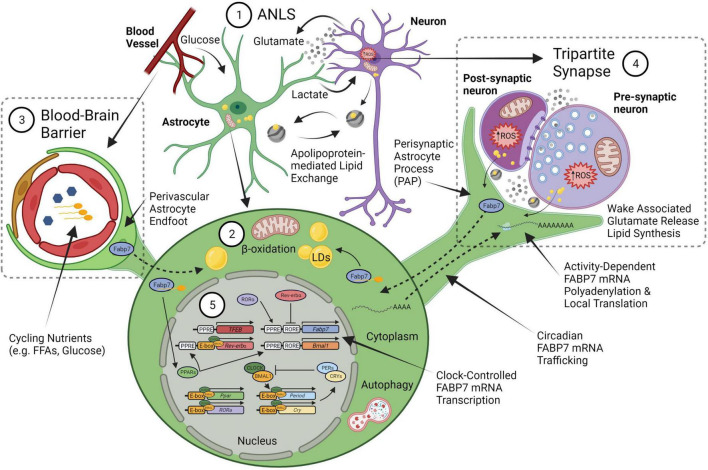
FIGURE 1.
An integrated neural-glial metabolic clock-sleep model. (1) The astrocyte-neuron lactate shuttle (ANLS) hypothesis proposes that activity-dependent glutamate release at synapses triggers astrocyte glucose uptake from blood, which is then converted to lactate and sent to neurons to support the increased metabolic demand. Sleep pressure (homeostasis) would be linked to the increased energy produced by this lactate in neuronal mitochondria from wake-associated glutamate release, which generates reactive oxygen species (ROS) production and subsequent lipid formation that are transferred back to glia via apolipoproteins (i.e., ApoE). (2) These lipids will bind fatty acid transport proteins, including FABP7, to form lipid droplets (LDs) in astrocytes. The lipid stores can be used as fuel in astrocyte mitochondria via β-oxidation to produce ketone bodies. (3) Astrocyte endfeet surround the brain vasculature as one of the cellular components of the blood brain barrier (BBB). Circulating nutrients and metabolic constituents such as free fatty acids (FFA) and glucose are taken up by astrocytes and used as energy for the brain. (4) This wake-associated glutamate release would also be tied to local translation of FABP7 mRNA in the fine perisynaptic astrocytic process (PAP) of the tripartite synapse, which consists of an astrocyte ensheathment along with pre- and post-synaptic neuronal compartments, to couple on-site FABP7 protein demand with newly synthesized lipids derived from local excitatory synaptic activity in neurons. (5) The timing of FABP7 mRNA expression is regulated by the circadian clock via Rev-erbα, a transcriptional repressor that binds to RORE cis-elements in the promoters of FABP7 gene and the core-clock transcriptional activator BMAL1. Following transcription, FABP7 mRNA is trafficked to PAPs where it is locally translated upon behavioral state-dependent changes in neural activity. Therefore, clock-controlled expression of FABP7 relays the circadian timing of sleep with changes in sleep pressure through mechanisms underlying local translation at PAPs. FABP7 may in turn feedback on transcription of the core-clock via nuclear localization and activation of peroxisome proliferator-activated receptor (PPARs), for example through FFAs, such as omega-3 polyunsaturated fatty-acids known to oscillate in the peripheral vasculature, or those from PAPs. PPAR—mediated transcription of Transcription Factor EB (TFEB) may in turn initiate autophagy (Soto-Avellaneda and Morrison, 2020), or alternatively, autophagy could be stimulated via other BMAL1-dependent mechanisms in astrocytes (McKee et al., 2023). Circadian FABP7 may also regulate BBB permeability over the course of the day to influence the transmission of peripheral signals and metabolic constituents to (or from) the brain. Created with BioRender.com.