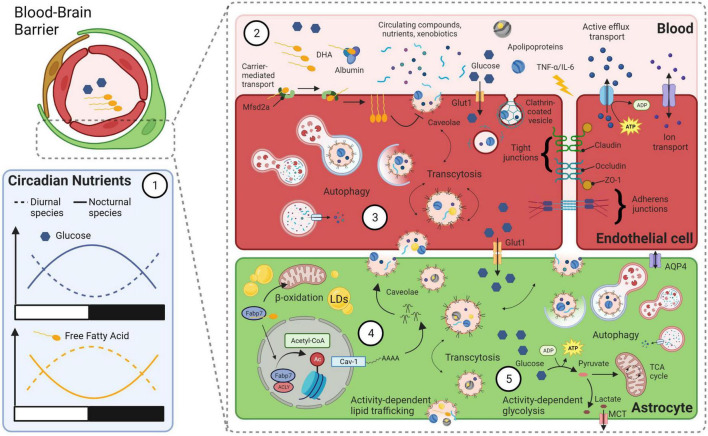
FIGURE 2.
A model linking bioenergetics with integrated clock-state-dependent fluctuations in metabolism and BBB permeability. (1) Diurnal and nocturnal species have diametrically opposed rhythms in circulating levels of glucose and free fatty acids (FFAs). Within species, levels of glucose and FFAs oscillate over the day in opposite phases. (2) Various transport mechanisms across the BBB can influence permeability and are closely tied to circadian rhythms and behavioral state. For example, circadian rhythms of circulating omega-3 fatty-acid, DHA, may bind to endothelial transport protein major facilitator superfamily domain containing 2a (Mfsd2a), which flips DHA from the outer-membrane to inner-membrane surface. This is known to block caveolae formation and transcytosis at the BBB (Andreone et al., 2017). Other lipid structures, such as apolipoproteins, may bind to receptors for endocytosis. Circadian changes in circulating glucose are trafficked across the BBB by transporters (e.g., Glut1). Circulating cytokines such as TNF-α and IL-6, whose levels increase with sleep loss, can disrupt tight junction proteins and increase BBB permeability. Clock-state dependent changes in efflux and ion transport can also occur at the BBB. (3) Autophagy of endocytosed materials and cellular damage following BBB disruption due to extended wakefulness may protect endothelial cells and increase their survival. (4) Astrocyte caveolae formation is driven by FABP7-mediated acetylation of histones via an ATP-citrate lyase (ACLY) interaction that increases nuclear Acetyl-CoA levels and activates the promoter of the caveolin-1 gene. Caveolae transcytosis in astrocytes may therefore be integrated between circadian oscillation in circulating DHA levels in the vasculature and the clock-controlled expression of FABP7. Astrocyte uptake of cycling lipids and DHA may in turn influence the formation of lipid droplets (LDs) and subsequent β-oxidation in mitochondria. (5) A balance between activity-dependent energy demands and circulating nutrient availability integrates the circadian clock with behavioral state in astrocytes. For example, local use-dependent neural activity will increase the ANLS, to convert glucose into lactate that is delivered to neurons from astrocytes via MCTs. This wake-dependent neural activity is coupled to increased lipid synthesis in neurons, that is taken up by astrocytes and stored in LDs for later use as fuel (see Figure 1). Metabolic byproducts of this bioenergetics pathway are cleared via autophagy, exocytosis and/or by the glymphatic system, via water channels such as Aquaporin 4 (AQP4). Created with BioRender.com.