Figure 1.
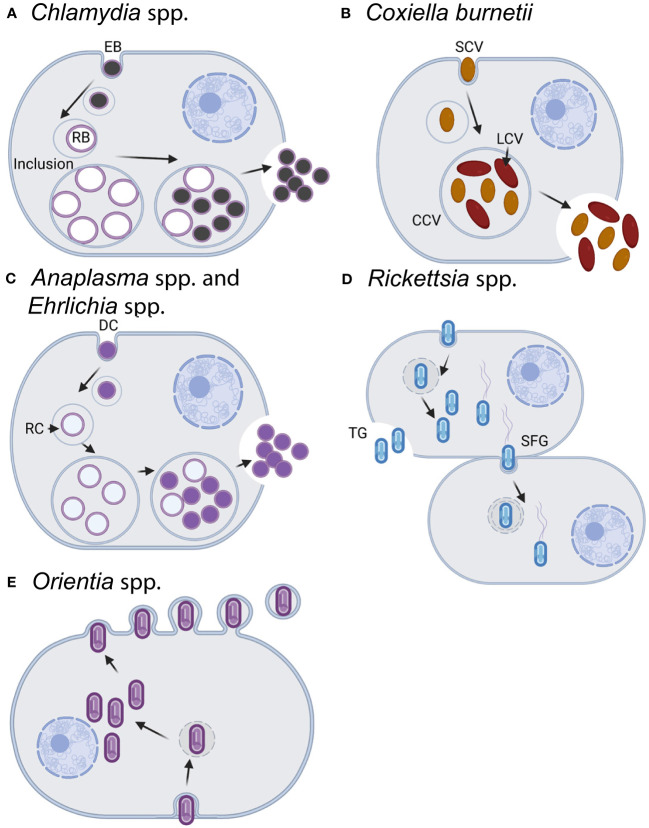
Infection and replication lifestyles of select obligate intracellular bacteria in the animal host. (A) Chlamydia spp. undergo a biphasic development cycle with the infectious elementary body (EB) internalized into the host cell (primarily epithelial cells) where it resides in a host-membrane-derived vacuole termed the inclusion (Abdelrahman and Belland, 2005; Elwell et al., 2016). The EB converts into the replicating reticulate body (RB), which divides and eventually differentiates back into the EB form. EBs exit the host cell through cell lysis or inclusion extrusion [not pictured, (Hybiske and Stephens, 2007)] ~36–72 h postinfection. (B) C. burnetii infects macrophages where it resides within an acidified phagolysosome-like vacuole known as the Coxiella-containing vacuole (CCV) (Minnick and Raghavan, 2012). Both forms of C. burnetii, the small-cell and large-cell variants (SCV/LCV), are infectious with the SCV being more environmentally stable and having a lower metabolic rate than the LCV. C. burnetii exits the cell through cell lysis, although the timing of natural release is uncertain. For experimental usage, cells are artificially lysed once the bacteria reach the stationary phase after ~5–6 days. C. burnetii may also be grown axenically in acidified citrate cysteine medium [ACCM, (Omsland et al., 2009; Vallejo Esquerra et al., 2017)]. (C) Both Anaplasma spp. and Ehrlichia spp. have an infectious dense-core cell form (DC) and a replicative reticulate cell form (RC) (Salje, 2021). Cell types infected vary by species and include neutrophils, monocytes/macrophages, erythrocytes, or endothelial cells. Following internalization in a membrane-bound vacuole, the DC form converts into the RC form which then replicate as a bacterial microcolony known as a morulae. RCs eventually convert back to the DC form and are released from the cell ~3 days postinfection by either lysis (pictured), exocytosis, or filopodia in an actin-dependent mechanism. The exit mechanisms can vary by species and the stage of infection. (D) Rickettsia spp. replicate within the cytoplasm and most commonly infect endothelial cells, although some species will also infect macrophages. Members of the typhus group (TG) exit the cell via cell lysis (~4–5 days postinfection), whereas the spotted fever group (SPG) members can enter into adjacent cells via actin-based motility (depicted) or be released following lysis of the host cell (~48 h postinfection). (E) Orientia spp. infect a wide variety of cells including endothelial cells, dendritic cells, monocytes, and macrophages (Salje, 2021). Bacteria replicate within the cytoplasm in close proximity to the nucleus and are released from the cell in a budding process ~4 days postinfection. Note that for all of the bacteria above, the cell types listed reflect natural infection reservoirs and that multiple permissive cell types may be used in the lab for bacterial growth and propagation. In addition to the listed differences in time for bacterial entry, replication, and release, the infectious burdens per host cell vary across species from as little as ~30–50 for the SPG Rickettsia to a few hundred for Chlamydia spp. Images created with BioRender.com.