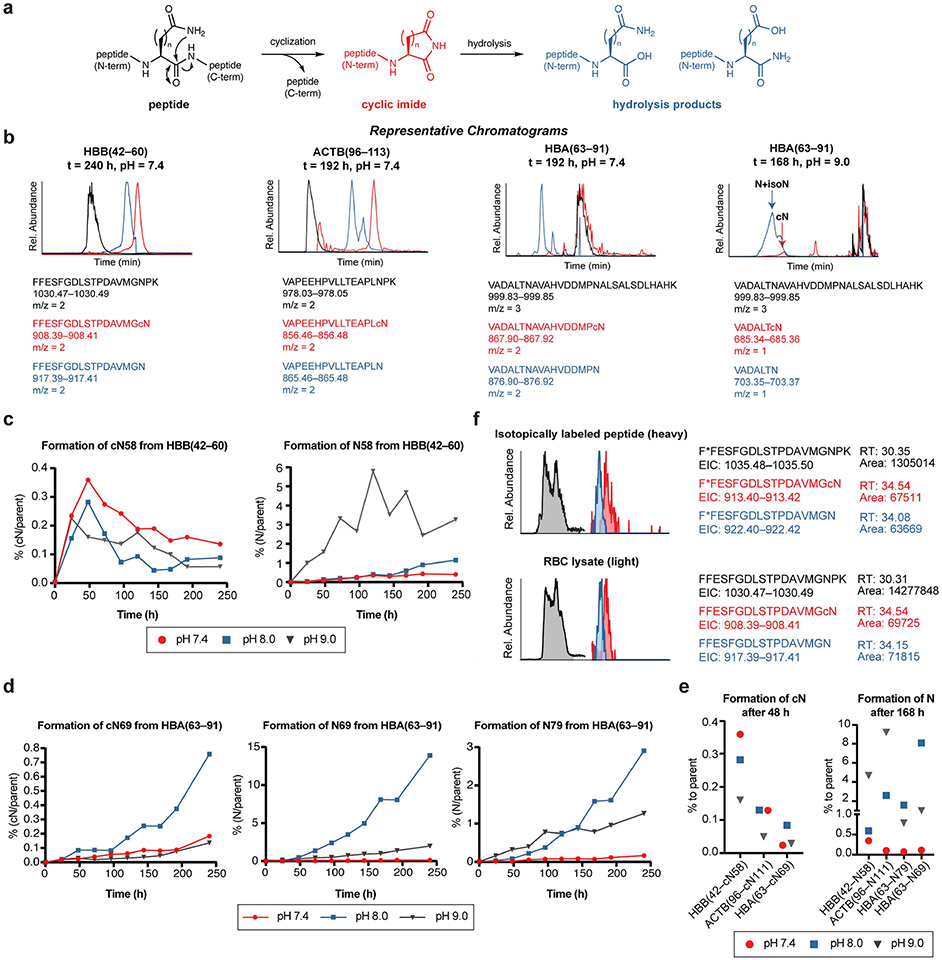
Extended Data Fig. 9 ∣. Analysis of C-terminal cQ/cN and Q/N modifications on synthetic peptides.
(a) Scheme of cyclic imide formation in a peptide and subsequent hydrolysis to afford the truncated C-terminal glutamine or asparagine fragments. (b) Overlay of representative extracted ion chromatograms of each peptide at the masses corresponding to parent peptide (black), cyclic imide fragment (red), and its hydrolyzed products (blue). The two constitutional isomers formed via hydrolysis of the cyclic imide fragment were not distinguished in our study. (c–d) In vitro time course for formation of the cyclic imide fragment and the hydrolysis products at the indicated position on the synthetic peptide after incubation. (e) Percentage of the formed cyclic imide fragment and the hydrolysis products at the indicated residue relative to the parent synthetic peptide at different pH. (f) Ion intensity chromatogram extracted for the masses of the corresponding species for RBC lysates and the mixture of isotopically labeled HBB(42*–60) containing the modifications. The RBC samples were spiked with the peptide mixture and ran on the selected ion monitoring mode to validate the overlap of retention times and amplify the signal of selected ions.