Abstract
Rift Valley fever (RVF) is an ecologically complex emerging arboviral disease that causes significant illness in both livestock and people. This review article is designed to assist the reader in understanding the varied aspects of RVF disease in animals and humans. The historical facets of RVF disease, including the evolution of human outbreaks, are presented and discussed. The different clinical presentations of human RVF disease and the underlying causes are then addressed. We explore the exposure and transmission potential of RVF in animals and people. In the concluding section, we discuss the historical role of RVF as a biological weapon. We conclude with an outline of the important unanswered questions for ongoing research into this important zoonotic disease.
1. Introduction
Rift Valley fever (RVF) is a veterinary disease of livestock in Africa, and as such it exemplifies the One Health concept, in which animal and human health are inextricably intertwined. Infection of domesticated livestock (sheep, cattle, and goats) with RVF virus (RVFV) causes a highly lethal illness that results in dire economic consequences in affected regions. Initially a disease of the Rift Valley in eastern Africa, RVFV has spread through continental Africa as well as to Madagascar and the Arabian Peninsula. The World Health Organization (WHO)’s first Workshop on Prioritization of Pathogens assigned RVF to the list of “severe emerging diseases with potential to generate a public health emergency, and for which no, or insufficient, preventive and curative solutions exist” (World Health Organization, 2015, 2017). RVF remains on the World Organization for Animal Health (OIE)’s list of notifiable animal diseases of concern World Organization for Animal Health (OIE), 2018. Veterinary and human vaccination strategies and development of therapeutic interventions are the key to limiting spread and alleviating disease burden. Both have recently been comprehensively reviewed (Dungu et al., 2018; Atkins and Freiberg, 2017) and are not discussed here. Instead, this review article is designed to assist laboratory researchers, clinicians, and public health practitioners in understanding the manifold aspects of RVF disease in animals and humans. We first provide a historical perspective on the disease, including a discussion of the changing recognition of human disease over time. The clinical presentations of human RVF disease and the potential mechanisms underlying different disease manifestations are then addressed. We then review evidence supporting exposure and transmission potential. In the concluding section, we discuss the historical and current role of RVF as a biological weapon. We conclude with an outline of the important unanswered questions for ongoing research into this important zoonotic disease.
2. Historical perspective on RVF disease
2.1. Identification and isolation of “enzootic hepatitis”
In 1930, R. Daubney and J.R. Hudson, working within the Division of Veterinary Research in Kenya, were alerted to an unusually high mortality in lambs on a farm near freshwater Lake Naivasha in the Rift Valley (Daubney and Hudson, 1931). Their initial investigation determined that these deaths were caused by a previously unrecognized disease of sheep and cattle (Daubney and Hudson, 1931). Illness in the lambs was abrupt; mortality often occurred within 24 h of disease onset. As Daubney described, “… the disease might entirely escape observation during life and the animal would simply be found dead in the morning” (Daubney and Hudson, 1933). Mortality of lambs was age-dependent; the highest mortality rates (up to 95%) occurred in 3–7 day old lambs (Daubney and Hudson, 1931). Signs of disease in adult animals included vomiting, diarrhea, and listlessness. In pregnant ewes, often the only sign of illness was abortion of the fetus, while the ewes themselves displayed few symptoms prior to being found dead. The high mortality in pregnant animals gave rise to the characteristic (and eerily descriptive) term ‘abortion storms’ used to describe the massive fetal mortality that accompany epizootic outbreaks of RVF (Fig. 1). The liver is the organ most affected in infected livestock. Upon gross examination, the liver was mottled in appearance and friable, with extensive necrotic lesions (Daubney and Hudson, 1933; Coetzer, 1977, 1982).
Fig. 1. Ecological cycle of Rift Valley fever.
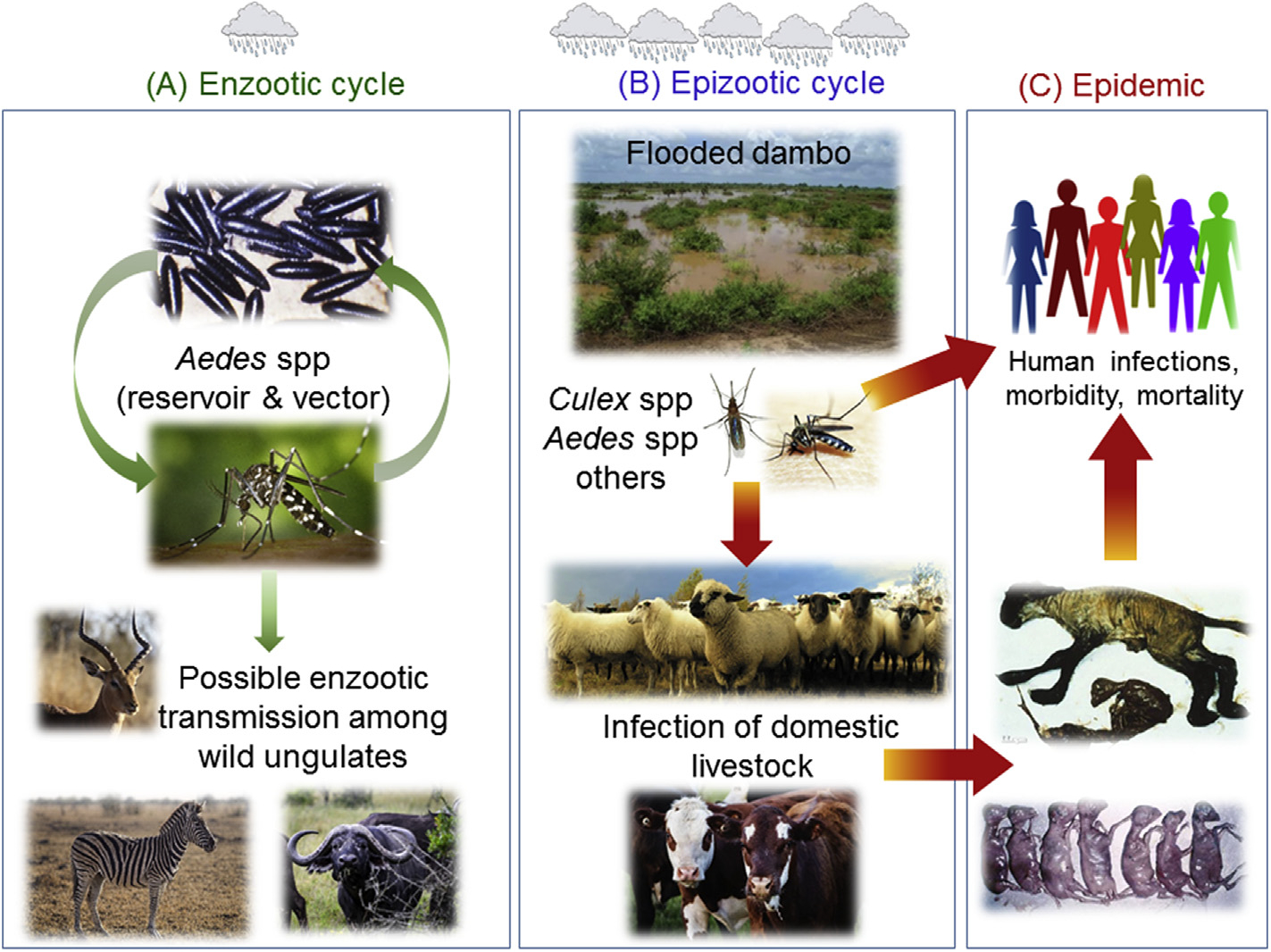
(A) In the enzootic cycle, RVFV is transmitted vertically within Aedes spp mosquito eggs; virus can remain infectious within dessicated eggs during dry seasons. Infected mosquitos can transmit the virus to wild ungulates, where it is thought to cause mild or inapparent illness. The role of wild animals as amplifying hosts is not clear. (B) The epizootic cycle commences in times of excess rainfall, whereby extensive floodplains (or dambos) lead to hatching of large numbers of mosquito eggs. Infected mosquitos feed off of livestock, causing abortion, illness, and death. Culex spp and other mosquitos are secondary vectors; they amplify epizootics by taking bloodmeals from viremic animals and transmitting the virus to other herds and humans over longer distances. (C) People become infected directly from mosquitos or by contact with infected animals. Contrary to most arboviruses, RVFV can infect a wide range of insect vectors, wild and domesticated animals, and humans, all of which contributes to the complexity of its ecological cycle.
Daubney and colleagues demonstrated that blood transferred from a sick to a healthy animal could transmit the disease; however, they detected no evidence, either naturally or experimentally, that the illness was naturally transmitted between animals. In an effort to control the epizootic, farmers transported sheep from the affected farm (at an altitude of 5500–6000 feet) to one located at a higher altitude (at 7000–8000 feet). This led to the subsidence of disease, which suggested that an intermediate vector was needed and that animals did not readily transmit the disease amongst themselves (Daubney and Hudson, 1931). In addition, field exposure experiments performed by Daubney and colleagues determined that mosquitoes or other insects that can be excluded by a common mosquito net were likely the cause of disease transmission, as housing sheep covered with a net prevented infection, whereas uncovered sheep were still susceptible (Daubney and Hudson, 1931, 1933). Furthermore, they determined that these vectors seemingly feed primarily at night, as cattle restricted to a day-time feeding did not become ill. Finally, an unusually large amount of rainfall that year (twice the normal annual precipitation level) coincided with the animal deaths (Daubney and Hudson, 1931). Collectively, these early observations pointed towards mosquitos as the likely vector. Later studies by others were able to isolate RVFV from a variety of mosquito species and demonstrate experimental transmission by mosquitoes (Smithburn et al., 1948, 1949a; Gear et al., 1955).
In hindsight, RVF likely existed in the Rift valley of Kenya for at least 20 years prior to its identification as a distinct disease in 1931. In 1913, for example, R.J Stordy, then the Chief Veterinary Officer in the Department of Agriculture for British East Africa (present-day Kenya), submitted a report detailing the occurrences of known livestock diseases during the previous year. The known diseases included rinderpest, East Coast fever, scabies, variola, and anthrax, among others. Stordy includes an intriguing section describing unnamed mortality among lambs (Montgomery, 1913):
“A mortality of 90 percent was recorded among lambs. In some cases the symptoms were very acute, and death occurred within a few hours. In others, the disease ran a more sub-acute or chronic course. In the acute form, the only symptoms shown were dullness, rapid respirations, collapse, and death within four hours. In post-mortem, the liver was found to be soft and friable and the kidneys congested. In the sub-acute or chronic form, the umbilicus was incompletely closed and swelling of the joints occurred. Investigation pointed to the disease resulting from the infection gaining entrance through the umbilicus.”
The first description of human disease was of illness among the scientists and veterinarians involved in the investigation of the 1931 epizootic, and then it was retrospectively identified among the local farmers of the affected herds during the outbreak (Daubney and Hudson, 1931). Human disease was confirmed by injection of a malaria patient at Native Hospital in Nairobi with filtered blood from a sick lamb (Daubney and Hudson, 1931; Findlay, 1932). The disease that developed in this patient and the other initial cases was described as ‘dengue-like fever’ or similar to influenza. One patient described “pains that developed in or near the joints extending from the base of the skull to the extremities (Daubney and Hudson, 1931).” Other common symptoms included fever, headache, abdominal pains, joint and muscle pains, photophobia, retro-orbital pain, vomiting, nosebleeds, and sweating (Daubney and Hudson, 1931; Findlay, 1932). The disease was first named ‘enzootic hepatitis’ due to the hepatic disease in animals, however it was quickly renamed ‘Rift Valley fever’ as a more accurate description of the febrile illness observed in humans (Daubney and Hudson, 1931).
After recognition of RVF as a new disease in 1931, Daubney and colleagues transported infectious blood samples to the Wellcome Bureau of Scientific Research in London where G.M. Findlay experimentally inoculated an assortment of animal species to examine the range of susceptibility (Findlay, 1932). Mice, rats, and hamsters were as susceptible as lambs, while other species (rhesus monkeys, cats, and rabbits) were less so.
2.2. Subsequent outbreaks
After the initial description of the disease in 1931, a lull in cases occurred (likely due to prolonged dry periods), with no cases reported in Kenya between 1936 and 1950. However, the 1950’s ushered in an era that, to date, has seen at least 11 epizootics in Kenya and neighboring east African countries during the past 60 years, each occurring an average of every 3.6 years (Table 1) (Murithi et al., 2011). By the 1950’s, RVF was found in South Africa, which has since endured 3 extensive epidemics: 1950–1951, 1974–1976, and 2010–2011. The 1950–51 South African outbreak resulted in the death of 100,000 sheep with 500,000 abortions as well as non-lethal febrile illness in man (Murithi et al., 2011). Numerous smaller epizootics or isolated cases continued, interspersed between each larger epidemic (Pienaar and Thompson, 2013).
Table 1.
List of Rift Valley Fever activity and estimated number of animal and human cases.
| Year | Location: | Humans | Animals | Additional Comments | References | ||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Cases | Deaths | Cases | Deaths | ||||
|
| |||||||
| 1930–1931 | Kenya | INDa | IND | IND | 5000 | Initial recognition of RVF as a distinct disease | (Daubney and Hudson, 1931) |
| 1950–1951 | Kenya/South Africa | IND | IND | 500,000 | 100,000 | (Murithi et al., 2011) | |
| 1974–1975 | South Africa | 110 | 7 | IND | IND | First documented fatal cases in humans | (McIntosh et al., 1980a; van Velden et al., 1977) |
| 1977–1979 | Egypt | 200,000 | 600 | IND | IND | First significant number of human fatalities | (Laughlin et al., 1979) |
| 1978 | Zimbabwe | IND | 3 | 80,000 | 20,000 | (Johnson et al., 1983) | |
| 1987 | Mauritania | IND | 220 | IND | IND | (Digoutte and Peters, 1989) | |
| 1997–1998 | East Africa | 89,000 | 478 | IND | IND | Centers for Disease Control and Prevention, 1998 | |
| 1998 | Mauritania | 400 | 6 | IND | IND | (Nabeth et al., 2001; Caminade et al., 2014) | |
| 2000–2001 | Saudi Arabia | 886 | 123 | > 10,000 | 1000 | First documented cases outside of Africa | (Madani et al., 2003) |
| 2000–2001 | Yemen | 1328 | 166 | 22,000 | 6000 | (Madani et al., 2003; World Health Organization, 2018c) | |
| 2003 | Egypt | 148 | 27 | IND | IND | (World Health Organization, 2018c) | |
| 2006–2007 | Kenya | 684 | 155 | > 4400 | 235 | (World Health Organization, 2018c) | |
| 2006–2007 | Somalia | 114 | 51 | IND | IND | (World Health Organization, 2018c) | |
| 2006–2007 | Tanzania | 309 | 142 | IND | 50,000 | (World Health Organization, 2018c; Sindato et al., 2015; Fyumagwa et al., 2011) | |
| 2007–2008 | Sudan | 747 | 230 | IND | IND | (Hassan et al., 2011; World Health Organization, 2018c) | |
| 2008–2009 | Madagascar | 418 | 17 | IND | IND | (World Health Organization, 2018c) | |
| 2010 | Mauritania | 63 | 13 | IND | IND | (Caminade et al., 2014; Faye et al., 2014; El Mamy et al., 2011) | |
| 2010–2011 | South Africa | 302 | 25 | 20,000 | 8581 | (Archer et al., 2013) | |
| 2012 | Mauritania | 41 | 18 | IND | IND | (Sow et al., 2014; World Health Organization, 2018c; Caminade et al., 2014) | |
| 2016 | China | 1 | 0 | IND | IND | Imported case originating from Luanda | (Liu et al., 2017) |
| 2016 | Niger | 266 | 32 | IND | IND | (World Health Organization, 2016) | |
| 2018 | South Sudan | ~20 | 4 | IND | IND | Currently ongoing | (World Health Organization, 2018b) |
| 2018 | Gambia | 1 | 1 | IND | IND | (World Health Organization, 2018a) | |
IND = indeterminate = the number of cases is not known, not provided, or unable to be estimated.
2.3. RVF as a serious human disease
While severe illness and lethality among livestock persisted in both Kenya and South Africa in the 1950s, the human dimension of RVF did not emerge until the 1970’s. Two interesting epidemics occurred during this period. First in South Africa, exceptionally heavy rainfall is believed to have caused a large outbreak of RVF between 1974 and 1976. Estimates of the number of livestock affected during this outbreak have not been recorded, however it is assumed that the numbers are equal to or larger than the 500,000 animals infected during 1950–51. Notably, in 1974, South Africa recorded the first human deaths due to RVFV (McIntosh et al., 1980a). Out of 110 laboratory-diagnosed human cases, there were 7 fatal cases with extensive liver damage and hemorrhagic fever, 15 cases of encephalitis, and 10 cases of retinitis with visual defects (van Velden et al., 1977).
Unexpectedly, a second large epidemic occurred in Egypt between 1977 and 1979 that changed the paradigm of RVF in humans. This is the first time the disease occurred outside of sub-Saharan Africa. The effect on the Egyptian economy was devastating, with almost half of all animals in the country affected by the disease. For the first time, significant human morbidity and mortality was observed (estimated at 200,000 human infections; 600 deaths) (Laughlin et al., 1979). The human clinical manifestations included disparate illnesses such as hemorrhagic disease, encephalitis, and ocular disease. RVFV most likely emerged in Egypt from importation of infected livestock or infected mosquitos either directly or indirectly from Sudan or Zimbabwe (Gad et al., 1986; Samy et al., 2017a; Bird et al., 2007).
2.4. Emergence of RVF in new locations
After the Egyptian outbreak, RVF was acknowledged as a potentially serious and lethal viral infection of humans. Subsequently, the disease was found in areas where it had not previously been seen, such as West Africa in the 1980’s (Senegal and Mauritania; 200 human deaths), Madagascar in the 1990’s (at least one human death), and Saudi Arabia and Yemen in the year 2000 (289 deaths) (Madani et al., 2003; Zeller et al., 1997; Saluzzo et al., 1987; Morvan et al., 1992; Digoutte and Peters, 1989; Baba et al., 2016) (Table 1).
The occurrence of RVF in Saudi Arabia and Yemen in the fall of 2000 is significant because this was the first time that RVF disease was found outside the continent of Africa (Madani et al., 2003). There were over 880 laboratory-confirmed human cases with 123 deaths during the 6-month long outbreak (Madani et al., 2003); this is most likely a vast underrepresentation of the total number of less severe human infections. Of the lab-confirmed patients, 18% had jaundice, 17% had neurological symptoms, and 7% had clinical signs of hemorrhagic fever. The mortality rate in patients with any of those severe symptoms was very high (45% for those with jaundice, 53% for those with CNS disease, and 65% for patients with hemorrhage). Evidence suggests the origin of this outbreak was an eastern African strain that was circulating in Kenya in 1997–1998 (Bird et al., 2007; Shoemaker et al., 2002; Samy et al., 2017b; Miller et al., 2002). The livestock trade from Sudan into Saudi Arabia is the likely source of importation of the virus. Muslims embark on an annual pilgrimage (Hajj) to Mecca, during which animals are ritually slaughtered. Animals used for slaughter during the Hajj are often imported from regions in Africa where RVFV is endemic. Retrospective analysis found that 17% of slaughterhouse workers in Mecca in 1999 were RVFV seropositive (Turkistany et al., 2001). Thus it is likely that the virus was imported within the 1997–1998 time frame, and a recent study suggests that a single introduction resulted in the outbreak on the Arabian Peninsula (Samy et al., 2017b). Rainfall in the outbreak area was much higher than normal in the year 2000, which likely provided a mechanism for emergence (Jupp et al., 2002). Despite the evidence of seropositive animals in Saudi Arabia since 2000, these animals likely originated from Africa, and therefore it is not known whether the virus is endemic on the Arabian Peninsula.
South Africa suffered another significant RVFV outbreak in 2010–2011, where there were close to 20,000 cases in animals, 302 laboratory-confirmed human cases, including 25 deaths (Pienaar and Thompson, 2013; Archer et al., 2013). In July of 2016, an imported case of RVFV was reported in China from a man who contracted the disease while working in Angola (Liu et al., 2017). Most recently, human cases of RVFV have occurred in Niger (266 human cases and 32 deaths have been suspected), South Sudan, and Gambia (World Health Organization, 2016, 2018a, 2018b). Taken together (Table 1), substantial epizootics and epidemics have occurred in at least 16 countries in Africa and the Arabian peninsula, with continuing reports of severe human illness and epizootics in livestock to date (World Health Organization, 2018a, 2018b).
3. Spectrum of human RVF disease
3.1. RVF as a febrile illness
Uncomplicated RVF occurs in most infected people. The illness begins with the sudden onset of headaches and body aches, followed quickly by fever lasting 3–5 days (Laughlin et al., 1979). Other generalized symptoms include abdominal pains, severe joint and muscle pains, vomiting, anorexia, weakness, nosebleeds, sweating, and constipation. In some patients, a biphasic fever occurs (Gear et al., 1951). While uncomplicated RVF is not lethal, severe headaches, joint pain in the back and extremities, and weakness are common and can last for a week or more (Daubney and Hudson, 1931; Gear et al., 1951). Clinical laboratory values are typically within normal ranges for blood chemistry and complete blood counts. In some cases, convalescence may last for several weeks.
3.2. Liver disease and hemorrhagic fever
Severe systemic disease occurs in humans at a low frequency (approximately 1–2% of cases). As with livestock disease, the liver is a major site of viral replication and tissue damage in people with severe RVF disease. In addition to the body aches and fever of typical RVF, patients progress to jaundice and possible hemorrhagic manifestations that include the presence of blood in urine/feces, vomiting of blood, purple skin rash, and gingival bleeding (Laughlin et al., 1979). Historically, RVF is most recognized for this disease presentation because of some similarities with Ebola hemorrhagic fever. Patients with severe RVF have extremely high levels of the liver enzymes alanine transaminase (ALT) and aspartate transaminase (AST), prolonged blood coagulation times, and decreased platelets (Madani et al., 2003; Liu et al., 2017; McElroy and Nichol, 2012). In patient samples from the Saudi Arabian outbreak in 2000, fatal cases had levels of ALT and AST that were 100–1000× above baseline, while patients with severe disease that survived had liver enzymes 10–100× above normal (McElroy and Nichol, 2012). Evidence of severe liver necrosis is seen upon autopsy (van Velden et al., 1977; Shieh et al., 2010; Abdel-Wahab et al., 1978). Renal failure is also commonly noted. The hepatic/hemorrhagic form of RVF is highly lethal, with patients succumbing within 1 week of symptoms (Meegan et al., 1981). During the 2000–2001 outbreak in Saudi Arabia, the mortality of patients exhibiting abnormal bleeding or jaundice was 65% and 45%, respectively (Madani et al., 2003).
3.3. RVF and ocular disease
Vision problems are one of the most common complications arising from RVFV infection, occurring in up to 10% of infected people, even in those with less severe disease presentations (Gear et al., 1951; Freed, 1951; Al-Hazmi et al., 2005; Arthur et al., 1993; Siam et al., 1980). A study of inpatients with severe systemic RVF and outpatients with mild disease reported ocular abnormalities at similar frequencies (Al-Hazmi et al., 2005). Visual defects occur 1–3 weeks after the onset of initial illness (Al-Hazmi et al., 2005; Siam et al., 1980). Ocular abnormalities can include photophobia, reduced vision, and blind spots. Uveitis, retinitis, and retinal hemorrhage are frequently documented. Vision defects can be permanent or may resolve in weeks to months. In the original report describing RVF, one patient “complained of headache and defective vision for some weeks afterwards (Daubney and Hudson, 1931).”
3.4. RVF neurological disease
While some individuals with severe systemic RVF display generalized neurological issues (headache, delirium) during the acute illness, onset of persistent and severe neurological problems can occur within days to weeks after resolution of the initial symptoms. Hallucination, disorientation, vertigo, excessive salivation, weakness, and/or paralysis are some of the neurological complications that can arise in RVF patients (McIntosh et al., 1980a; van Velden et al., 1977; Madani et al., 2003). Decerebrate posturing is frequently associated with patients that subsequently succumb to disease, while patients with hemiparesis may not recover completely several months after illness (Laughlin et al., 1979), Pleocytosis of the cerebrospinal fluid (CSF) is common (McIntosh et al., 1980a; Laughlin et al., 1979). During the 2000 Saudi outbreak, 53% of patients with CNS complications died, although it is unclear as to the exact cause of death in these individuals (Madani et al., 2003).
3.5. Factors underlying different disease outcomes
This spectrum of human RVF illnesses has occurred in virtually every outbreak since the 1970’s (Madani et al., 2003; Shieh et al., 2010; Mohamed et al., 2010; Hassan et al., 2011; Sow et al., 2014). The reasons behind the seemingly sudden increase in human illness in Egypt and South Africa in the 1970’s are unknown. One reason may be that the severe forms of RVF occurred in the earlier outbreaks but were not recognized as such. Other reports suggest that genetic evolution and possible reassortment of the virus may have led to the increased occurrence of severe RVF in humans (Baba et al., 2016). Several recent studies suggest that reassortment has occurred, albeit at a very low frequency (Samy et al., 2017b; Liu et al., 2017; Grobbelaar et al., 2011; Meegan, 1979; Bird et al., 2008; Freire et al., 2015; Sall et al., 1999). The relationship between genetic evolution or possible reassortment and increased viral virulence is not known.
Why some individuals develop more severe complications than others is difficult to ascertain, however co-infection with another microbial agent or chronic disease may be contributing factors. For instance, those who had a recent illness are more likely to have a severe case of RVF, although ‘recent illness’ was not defined specifically (LaBeaud et al., 2015). A few studies have examined the effect of human immunodeficiency virus (HIV) infection on co-infection with RVFV. In Tanzania in 2007, the case fatality rate of patients with RVF and HIV was significantly higher (75%) than HIV-negative RVF-infected patients (13%) (Mohamed et al., 2010). A small study during the South African outbreak in 2010–2011 revealed that of 3 HIV+ patients in the study, all developed RVF encephalitis (Jansen van Vuren et al., 2015). While the studies are limited, HIV infection appears to be associated with a higher occurrence of fatality and possibly neurological disease.
Increased susceptibility to development of severe RVF may be caused by a genetic predisposition for the disease. One recent study postulated that genetic polymorphisms in the innate immune system may contribute to severe RVF outcomes (Hise et al., 2015). Single nucleotide polymorphism (SNP) analyses on 363 individuals (117 RVFV +; 246 controls) from an RVF-endemic area in Kenya found that seven genes in the innate immune system were associated with severe disease manifestations using a single major locus model. SNPs within toll-like receptor 3 (TLR-3), TLR-7, TLR-8, retinoic acid-inducible gene-I (RIG-I), myeloid differentiation primary response 88 (MYD88), TIR-domain-containing adapter-inducing interferon-β (TRIF), and mitochondrial antiviral signaling protein (MAVS) genes were associated with more severe disease (Hise et al., 2015).
4. Exposure and transmission potential
4.1. Human exposure routes
A unique feature of RVFV outbreaks is the potential for people to be exposed to RVFV through different routes, namely by mosquito bite or contact exposure from sick animals. Mosquitos are the reservoir and vector for transmission of RVFV to animals and people (Fig. 1). The frequency of human infections by mosquito or contact exposure is not known. The Chinese man who acquired RVFV infection in Angola was likely exposed by mosquitos because he had no contact with animals (Liu et al., 2017), but in many cases the exposure route is not as clear because people are around both mosquitos and sick animals. Domesticated animals are considered amplifying hosts (Ahmed Kamal, 2011); that is, they exhibit high viremia and amplify transmission through mosquitoes as well as people handling infectious animal tissues. There are numerous reports in the literature that trace human infection to handling organs, tissues, and blood from infected animals (McIntosh et al., 1980a; van Velden et al., 1977; Gear et al., 1951; LaBeaud et al., 2015; Anyangu et al., 2010). Factors associated with higher likelihood of RVFV infection include occupations such as a herdsman or farmer; consuming, handling, or slaughtering sick animals; and sleeping or living in close proximity to animals (LaBeaud et al., 2015; Anyangu et al., 2010). These studies also found that more severe disease was associated with handling aborted livestock fetuses.
The index case of an outbreak in Tanzania was a herdsman who reported butchering a sick goat a few days prior (Mohamed et al., 2010). Exposure in such an instance can occur through mucous membranes of the eyes, nose, and mouth, or through cuts and abrasions in the skin. Also plausible is the potential for inhalation of aerosolized particles from blood and tissue. Early studies demonstrated that RVFV is highly stable and infectious in aerosol form (Miller et al., 1963). During the Egyptian outbreak in 1977, a group of 6 investigators collecting samples became exposed to RVFV during the slaughter of a severely sick animal by the traditional Islamic throat-cutting technique. Blood spurting from the animal was collected for analysis and was later determined to have a very high titer of virus (Hoogstraal et al., 1979). All 6 investigators became infected but none had physical contact with the animal tissue or blood during the slaughtering, and the presumed infection route was likely inhalation of blood droplets created during the slaughter. The outcome of the 6 exposed individuals was not stated but it is assumed that they survived without significant complications.
4.2. Accidental infections
Prior to 1949, at least 25 accidental infections occurred in people working with the virus in the laboratory or in experimental settings (Smithburn et al., 1949b; Francis and Magill, 1935; Kitchen, 1934; Schwentker and Rivers, 1934; Sabin and Blumberg, 1947). In 1947, Albert B. Sabin and Richard W. Blumberg remarked “… there have been accidental human infections in practically every laboratory in which this virus has been studied” to that date (Sabin and Blumberg, 1947). Sabin, the future developer of the oral polio vaccine, describes his own lab-acquired infection with RVFV. Sabin began working with what he initially thought was a mouse-adapted strain of dengue virus but turned out to be RVFV. Without the advantage of having modern biosafety equipment or protection, he states that he used a mortar to grind mouse brain tissue for injection into mice, at which time “… contamination of the skin or aspiration of droplets of virus might have occurred.” He became ill 6 days later, when he abruptly awoke with a severe headache, followed by a fever later in the day. Severe muscle, bone, and joint aches lasted for 2 days. He returned to work in the laboratory 5 days after onset of illness. Mice inoculated with blood drawn from him shortly after onset of symptoms died from severe hepatic disease. Out of these initial reports on accidental lab infections, only one person died from complications indirectly related to RVFV (Schwentker and Rivers, 1934). While these accidental infections led to early knowledge about the disease course in humans, they also contributed to the original assumption that the virus was non-lethal in humans.
4.3. Transmission limitations
Although animal-to-human transmission is a significant mode of exposure of people, the frequency of transmission between animals remains unclear. The initial investigation of the 1930 outbreak by Daubney and colleagues (Daubney and Hudson, 1931) involved serial inoculations of blood from a naturally-infected lamb administered to uninfected sheep which demonstrated transmission. Naive sheep cohoused with the experimentally infected animals did not become infected, and these uninfected sheep were later shown to be susceptible to experimental inoculation. Other studies also failed to demonstrate contact spread in experimentally inoculated sheep and lambs (Easterday et al., 1962). On the other hand, several studies identified cases of horizontal transmission between sheep in an experimental setting (Busquets et al., 2010; Harrington et al., 1980; Yedloutschnig et al., 1981). More recently, Wichgers Schreur et al., (2016) observed that co-housing either naïve immunocompetent or immunocompromised (per treatment with dexamethasone) lambs with RVFV-infected lambs did not result in horizontal transmission, while direct inoculation confirmed that the lambs were susceptible to RVFV infection. Release of infectious virus in urine, feces, and milk is variable, although there are reports of shedding in milk from infected cows. Shedding of virus in respiratory secretions has been shown in experimentally inoculated animals (Busquets et al., 2010) and from infected people (Francis and Magill, 1935), but it is uncertain if this is a natural mode of transmission among animals.
Unlike other viral diseases such as Ebola, RVFV is not transmitted from person to person, thus humans are dead-end hosts. A study of 4 hospitals in Saudi Arabia during the 2000 outbreak showed that nosocomial transmission did not occur and that standard precautions including gloves, face masks, and gowns are suitable to protect healthcare workers caring for RVF patients (Al-Hamdan et al., 2015). Nosocomial transmission was not reported during the Egyptian outbreak either (Bres, 1981). Because nosocomial transmission is theoretically possible, personal protective measures are recommended to prevent exposure to blood and bodily fluids of suspected patients. For care of suspected or confirmed RVF patients in the United States, the Centers for Disease Control and Prevention recommends following the same guidelines for healthcare workers developed and updated for the Ebola outbreak in 2014 (Centers for Disease Control, 2014). The WHO recommends Standard Precautions that include the use of gloves, protective gown, face mask, and eye protection (World Health Organization, 2018c).
4.4. Human miscarriage and vertical transmission
Despite the large incidence of fetal loss in livestock infected with RVFV (Meegan, 1979; Hoogstraal et al., 1979), the effect of RVF infection on the human fetus has been less obvious. Initially, two studies performed in the 1980’s did not find a correlation between RVF infection and miscarriage (Abdel-Aziz et al., 1980; Niklasson et al., 1987). One such study determined that women who were RVFV-seropositive (IgG) did not have a higher incidence of miscarriage or still-birth (Niklasson et al., 1987). RVFV seropositivity in this study suggested past history of infection and not necessarily infection during pregnancy. More recently, a study performed in Port Sudan between June 30, 2011 and Nov 17, 2012 showed a significant association between laboratory-confirmed RVFV infections during pregnancy and an increased risk for miscarriage (Baudin et al., 2016). Women with acute RVFV infection during pregnancy had a higher rate of miscarriage (54%) compared to those without RVF (12%; p < 0.0001). In particular, RVFV infection was associated with late miscarriage in the second and third trimesters. Although this study did not analyze the presence of RVFV in fetal tissue, vertical transmission has been confirmed in 2 independent case reports discussed below (Arishi et al., 2006; Adam and Karsany, 2008).
In one case report, a Saudi Arabian woman delivered a full-term baby at home. The parents presented the baby to the hospital several days later with fever, lethargy, jaundice, and respiratory distress. The infant had an enlarged liver, severe thrombocytopenia, elevated liver enzymes, and prolonged coagulation times (Arishi et al., 2006). Serum was positive for RVFV IgM. Despite receiving fluids and blood transfusions, the infant developed severe anemia and disseminated intravascular coagulation. Six days after birth, he exhibited hemorrhagic manifestations (rash and excessive bleeding) and succumbed to the illness. In the 2 weeks prior to the boy’s birth, a number of close family members became ill, one of whom died of lab-confirmed RVF. Four days prior to delivery, the mother reported symptoms consistent with RVF, including fever, muscle aches, and headaches. The family owned sheep and goats that were affected by the RVF epidemic.
A second case of vertical transmission was documented in 2008, when a pregnant Sudanese woman developed fever and headache symptoms. Ten days after the symptoms began (38 weeks gestation), she delivered a baby boy with a skin rash and enlarged liver. Serum from both the mother and infant tested positive for RVFV-specific IgM (Adam and Karsany, 2008). The clinical outcome of this infant is unknown. While data are suggestive that RVF can cause miscarriage and vertical transmission, more detailed epidemiological and mechanistic studies are clearly needed.
5. RVF as an emerging disease
The capacity for RVFV to emerge outside of Africa was demonstrated in 2000 with the outbreak on the Arabian Peninsula. This event has led to concern over the potential further emergence of RVF to Europe or the Americas akin to the recent spread of other arboviruses, namely West Nile, chikungunya, and Zika viruses. As Moreno-Madrinan and Turell discuss, the zoonotic life cycle of West Nile virus was a major factor in its rapid spread and subsequent persistence in the United States compared to anthroponotic viruses like chikungunya and Zika, in which local persistence remains relatively rare (Max and Michael, 2018). Establishment of RVFV in animals in the United States would likely contribute to persistence and make control or eradication more challenging.
RVFV has been isolated from and/or shown to infect mosquitoes spanning across seven genera (Aedes, Anopheles, Coquillettidia, Culex, Eretmapoites, Mansonia and Ochlerotatus), as well as sand flies (Smithburn et al., 1948, 1949a; Gear et al., 1955; Turell and Perkins, 1990; Fontenille et al., 1995, 1998; Turell et al., 1996, 2008; Rolin et al., 2013; Linthicum et al., 2016; McIntosh, 1972; McIntosh et al., 1980b; Himeidan et al., 2014). The fact that the virus has adapted to such a wide range of insect vectors is unusual among arboviruses and contributes to the overall complex ecological cycle (Fig. 1). A number of studies determined that mosquitoes in North America and Europe could be competent vectors for RVFV (Turell et al., 2013; Brustolin et al., 2017; Vloet et al., 2017), and in particular, Aedes aegypti have been increasing in numbers and distribution in the United States since the early 1970’s (Max and Michael, 2018). Outbreaks of RVF in Madagascar raise concerns that the virus can perpetuate in more temperate climates (Morvan et al., 1991), which would expand the potential range.
Importation of infected mosquitoes or livestock, air travel or cargo are all potential risks for introduction of RVFV into naïve “virgin soil” locations such as the United States (Rolin et al., 2013). However, modeling studies are still unclear whether the virus would become enzootic if introduced by natural mechanisms (Barker et al., 2013). Algorithms have been developed to assess likely entry points of RVFV or the “risk season” in which the virus is most likely to enter the United States (Konrad and Miller, 2012; Kasari et al., 2008). A few of the locations with the highest likelihood of entry include regions with international airports or seaports in which a high volume of African visitors utilize (New York City, Washington DC, Houston, Baltimore, Atlanta, and Philadelphia) and states with a high population of African immigrants (California, Florida, New York, Texas, Maryland) (Kasari et al., 2008).
Multiple variables (temperature, rainfall, altitude, population, agricultural setting) have been evaluated to determine likelihood of establishment, yet each variable can be location-dependent. For instance, heavy rainfall in Africa often leads to epizootic events due to rehydration of mosquito eggs and subsequent hatching (Daubney and Hudson, 1931; Davies et al., 1985), whereas heavy rainfall does not seem to promote other epizootic events in the temperate United States (Nasci and Moore, 1998). Using a degree-day model that also takes into account the livestock population and predicted entry points into the United States, Konrad and colleagues predicted the time-periods, or seasons, and locations with the highest risk for establishment (Konrad and Miller, 2012). Temperature (average temperature maximum and minimum over a ten year period) was used as a limiting factor in this study because establishment of arboviruses within the vector is a temperature-dependent biological process (Reisen et al., 2006). Overall, the northern states were at risk in July and August, whereas risk dates span through the spring and fall for a majority of the warmer, southern states. Southern Texas and Florida were at risk for most of the year, (up to 295 days or 365 days, respectively). Other areas had zero risk, such as in the higher elevations of the Rocky Mountains, an area that is not conducive to mosquito populations (Eisen et al., 2008). One region of most concern was between Baltimore and New York City, as this region contains a large livestock population and multiple international airports. Climate change should also be considered when evaluating risk seasons and locations, as with increased temperature, the season length will likely increase.
If the virus was able to become endemic in animals in the United States or European countries, the immediate costs in terms of human illness, loss of animals, disruption of animal supply chains, and mitigation efforts are difficult to estimate but would likely be significant. As an example, in 2002 alone, North Dakota, a state with a large horse industry, spent $781,203 on medical costs and $400,000 on community awareness campaigns, sample collection and testing for West Nile virus positive horses, birds and mosquitos (Ndiva Mongoh et al., 2008). Further economic losses would come due to control policies and international trade restrictions imposed on US livestock to other countries (Hartley et al., 2011). The U.S. Department of Homeland Security supports development and use of emergency veterinary vaccines in the event of an epizootic in the U.S. (Food and Agriculture Organization of the United Nations, 2014).
6. RVF as a historical bioweapon
During World War II, many countries explored a variety of pathogens for their ability to be used as biological weapons (Bryden, 1989; Harris, 1994; Ellis and Moon, 2006; Leitenberg and Zilinskas, 2012; James Martin Center for Nonproliferation Studies, 2008; Avery, 2006; International Institute for Peace and Conflict Research, 1971). These included anti-crop and anti-animal pathogens in addition to diseases affecting people. In 1941, the Bacteriological Warfare Committee, comprised of American, British, and Canadian civilian scientists, was established (Endicott and Hagerman, 1998). Concerned about reports on the activities in Japan and Germany, the committee recommended that all offensive and defensive options be explored, including human, animal, and plant diseases. At the top of the committee’s initial list was Rinderpest and RVF, both diseases of livestock. Research on RVFV continued during the Cold War (Ellis and Moon, 2006; Leitenberg and Zilinskas, 2012; James Martin Center for Nonproliferation Studies, 2008; Avery, 2006; International Institute for Peace and Conflict Research, 1971; Endicott and Hagerman, 1998).
In the United States, research on RVF as a biological weapon occurred under Eisenhower’s administration in the 1950s, and concluded when Nixon ended the offensive biological weapon program in 1969 and the United States signed the Biological Weapons Convention in 1972. During initial investigations, RVF was considered a non-lethal incapacitating weapon against people. As discussed in previous sections, the early strains of the virus were only known to cause febrile illness in humans that resembled dengue or influenza. For this reason, RVFV was initially designed to be used for crippling the economic system of a targeted country through infection of the livestock. One appeal for such a weapon was the ability to cause as much economic damage as possible to an area without risk of human life.
Operation Whitecoat (1954–1973) was the United States’ biodefense medical research program in which human volunteers were used to test vulnerability of people to realistic biological weapons scenarios, which included aerosol exposure to a variety of biological agents (Anderson, 2013; Jones, 2016). Following the Nuremberg Code, data were collected on the infective doses, serological responses, clinical effects, and the effectiveness of drugs and vaccines. A formalin-inactivated RVFV vaccine (NDBR 103 RVFV) was evaluated for immunogenicity and efficacy in volunteers. After these initial trials, this vaccine was used for years to protect laboratory workers (Niklasson, 1982; Kark et al., 1982; Eddy et al., 1981). During the 1977 outbreak in Egypt where the highly virulent ZH501 strain emerged to cause lethal disease in people, U.S. naval sailors working with patient samples containing the virus at Naval Medical Research Unit No. 3 (NAMRU-3) in Cairo were given the NDBR 103 experimental vaccine (Peters, 1997). A next generation version of a formalin inactivated vaccine, the Salk Institute-Government Services Division (TSI-GSD) 200, was developed by U.S. Army. To this day, TSI-GSD 200 has been used to vaccinate lab workers through the Army’s Special Immunizations Program (SIP) (Special Immunizations Program, 2011).
Despite the fact that both the United States Patriot Act of 2002 and the United Kingdom’s Anti-terrorism, Crime and Security Act of 2001 listed RVFV as a potential bioterrorism agent, there are conflicting opinions as to the likelihood that RVFV would be used as a bioweapon and the benefit of listing RVFV as such (Mandell and Flick, 2011; Dar et al., 2013a). Dar et al., (2013b) explained that considering RVFV as a potential bioterrorism risk against human health is unprecedented as human-to-human transmission is absent and the risk of mortality due to severe disease is low. Instead, this editorial states that classification of RVF as a Select Agent with potential for intentional misuse has resulted in decreased international and national scientific collaborations, resulting in reduced scientific progress in the field that would be needed in the event of naturally occurring epidemics. Conversely, others note the ability of RVFV to be propagated easily in vitro, worldwide distribution of vectors, a long history of accidental infections, and the potential for genetic enhancement of virulence means that RVFV remains of great concern for misuse (Mandell and Flick, 2011).
7. Unanswered questions and future directions
This review article summarized some of the most intriguing historical and current aspects of Rift Valley fever. Many additional unanswered question are worth addressing in order to have a more comprehensive understanding of RVF disease. Certainly the underlying mechanisms that lead to different disease outcomes warrant further study. Does co-infection with another infectious agent commonly found in RVF-endemic areas, such as malaria or tuberculosis, affect clinical outcome? How does HIV-mediated immunosuppression affect the infection rate with RVFV and subsequent clinical manifestations? In addition, there are still many unanswered questions about the dynamics of human epidemics. Has RVFV truly become endemic on the Arabian Peninsula since the 2000 epidemic? What is the frequency of human infections as a result of mosquito bite vs contact exposure from animals? Does infection route affect disease presentation or severity? Why is RVFV rarely transmitted by contact between animals and not at all between humans? Finally, what (if any) role does RVFV have in vertical transmission and human miscarriage? Sporadic cases of animal and human disease continue to occur with larger outbreaks every few years. Given the likelihood of emergence beyond the current locations and its potential threat to animals, people, and the economy, continued research into this fascinating virus is merited.
Footnotes
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.antiviral.2018.05.009.
References
- Abdel-Aziz AA, Meegan JM, Laughlin LW, 1980. Rift Valley fever as a possible cause of human abortions. Trans. R. Soc. Trop. Med. Hyg. 74 (5), 685–686. [DOI] [PubMed] [Google Scholar]
- Abdel-Wahab KS, El Baz LM, El-Tayeb EM, Omar H, Ossman MA, Yasin W, 1978. Rift Valley Fever virus infections in Egypt: pathological and virological findings in man. Trans. R. Soc. Trop. Med. Hyg. 72 (4), 392–396. [DOI] [PubMed] [Google Scholar]
- Adam I, Karsany MS, 2008. Case report: rift Valley Fever with vertical transmission in a pregnant Sudanese woman. J. Med. Virol. 80 (5), 929. [DOI] [PubMed] [Google Scholar]
- Ahmed Kamal S, 2011. Observations on rift valley fever virus and vaccines in Egypt. Virol. J. 8, 532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hamdan NA, Panackal AA, Al Bassam TH, Alrabea A, Al Hazmi M, Al Mazroa Y, Al Jefri M, Khan AS, Ksiazek TG, 2015. The risk of nosocomial transmission of Rift Valley fever. PLoS Neglected Trop. Dis. 9 (12), e0004314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hazmi A, Al-Rajhi AA, Abboud EB, Ayoola EA, Al-Hazmi M, Saadi R, Ahmed N, 2005. Ocular complications of Rift Valley fever outbreak in Saudi Arabia. Ophthalmology 112 (2), 313–318. [DOI] [PubMed] [Google Scholar]
- Anderson AO. Infectious Disease and the Ethics of Human Volunteers in respect to the Whitecoat Project [8/25/15]. Available from: http://usarmywhitecoat.com/wp-content/uploads/2013/12/1954-73-Op-Whitecoat-ID-Res-No-Video-press.pdf. [Google Scholar]
- Anyangu AS, Gould LH, Sharif SK, Nguku PM, Omolo JO, Mutonga D, Rao CY, Lederman ER, Schnabel D, Paweska JT, Katz M, Hightower A, Njenga MK, Feikin DR, Breiman RF, 2010. Risk factors for severe Rift Valley fever infection in Kenya, 2007. Am. J. Trop. Med. Hyg. 83 (2 Suppl. l), 14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer BN, Thomas J, Weyer J, Cengimbo A, Landoh DE, Jacobs C, Ntuli S, Modise M, Mathonsi M, Mashishi MS, Leman PA, le Roux C, Jansen van Vuren P, Kemp A, Paweska JT, Blumberg L, 2013. Epidemiologic investigations into outbreaks of Rift Valley fever in humans, South Africa, 2008–2011. Emerg. Infect. Dis. 19 (12), 1918–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arishi HM, Aqeel AY, Al Hazmi MM, 2006. Vertical transmission of fatal Rift Valley fever in a newborn. Ann. Trop. Paediatr. 26 (3), 251–253. [DOI] [PubMed] [Google Scholar]
- Arthur RR, el-Sharkawy MS, Cope SE, Botros BA, Oun S, Morrill JC, Shope RE, Hibbs RG, Darwish MA, Imam IZ, 1993. Recurrence of Rift Valley Fever in Egypt. Lancet 342 (8880), 1149–1150. [DOI] [PubMed] [Google Scholar]
- Atkins C, Freiberg AN, 2017. Recent advances in the development of antiviral therapeutics for Rift Valley fever virus infection. Future Virol. 12 (11), 651–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery D, 2006. The Canadian biological weapons program and the tripartite alliance. In: Wheelis M, Rozsa L, Dando M (Eds.), Deadly Cultures: Biological Weapons since 1945. Harvard University Press, Cambridge, MA, pp. 84–107. [Google Scholar]
- Baba M, Masiga DK, Sang R, Villinger J, 2016. Has Rift Valley fever virus evolved with increasing severity in human populations in East Africa? Emerg. Microb. Infect. 5, e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker CM, Niu T, Reisen WK, Hartley DM, 2013. Data-driven modeling to assess receptivity for rift valley Fever virus. PLoS Neglected Trop. Dis. 7 (11), e2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudin M, Jumaa AM, Jomma HJE, Karsany MS, Bucht G, Naslund J, Ahlm C, Evander M, Mohamed N, 2016. Association of Rift Valley fever virus infection with miscarriage in Sudanese women: a cross-sectional study. Lancet Glob Health 4 (11), e864–e871. [DOI] [PubMed] [Google Scholar]
- Bird BH, Khristova ML, Rollin PE, Ksiazek TG, Nichol ST, 2007. Complete genome analysis of 33 ecologically and biologically diverse Rift Valley fever virus strains reveals widespread virus movement and low genetic diversity due to recent common ancestry. J. Virol. 81 (6), 2805–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird BH, Githinji JW, Macharia JM, Kasiiti JL, Muriithi RM, Gacheru SG, Musaa JO, Towner JS, Reeder SA, Oliver JB, Stevens TL, Erickson BR, Morgan LT, Khristova ML, Hartman AL, Comer JA, Rollin PE, Ksiazek TG, Nichol ST, 2008. Multiple virus lineages sharing recent common ancestry were associated with a Large Rift Valley fever outbreak among livestock in Kenya during 2006–2007. J. Virol. 82 (22), 11152–11166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bres P, 1981. Prevention of the spread of Rift Valley Fever from the african continent. Contrib. Epidemiol. Biostat. 3, 178–190. [Google Scholar]
- Brustolin M, Talavera S, Nunez A, Santamaria C, Rivas R, Pujol N, Valle M, Verdun M, Brun A, Pages N, Busquets N, 2017. Rift Valley fever virus and European mosquitoes: vector competence of Culex pipiens and Stegomyia albopicta (= Aedes albopictus). Med. Vet. Entomol. 31 (4), 365–372. [DOI] [PubMed] [Google Scholar]
- Bryden J, 1989. Deadly Allies: Canada’s Secret War 1937–1947. McClelland & Stewart, Inc., Toronto, Ontario. [Google Scholar]
- Busquets N, Xavier F, Martin-Folgar R, Lorenzo G, Galindo-Cardiel I, del Val BP, Rivas R, Iglesias J, Rodriguez F, Solanes D, Domingo M, Brun A, 2010. Experimental infection of young adult European breed sheep with Rift Valley fever virus field isolates. Vector Borne Zoonotic Dis. 10 (7), 689–696. [DOI] [PubMed] [Google Scholar]
- Caminade C, Ndione JA, Diallo M, MacLeod DA, Faye O, Ba Y, Dia I, Morse AP, 2014. Rift Valley Fever outbreaks in Mauritania and related environmental conditions. Int. J. Environ. Res. Publ. Health 11 (1), 903–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 1998. Rift valley Fever–east Africa, 1997–1998. MMWR Morb. Mortal. Wkly. Rep. 47 (13), 261–264. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Viral Hemorrhagic Fevers (VHFs): Information for Healthcare Workers 2014. [4/18/18]. Available from: https://www.cdc.gov/vhf/abroad/healthcare-workers.html.
- Coetzer JA, 1977. The pathology of Rift Valley fever. I. Lesions occurring in natural cases in new-born lambs. Onderstepoort J. Vet. Res. 44 (4), 205–211. [PubMed] [Google Scholar]
- Coetzer JA, 1982. The pathology of Rift Valley fever. II. Lesions occurring in field cases in adult cattle, calves and aborted foetuses. Onderstepoort J. Vet. Res. 49 (1), 11–17. [PubMed] [Google Scholar]
- Dar O, McIntyre S, Hogarth S, Heymann D, 2013a. Rift Valley fever and a new paradigm of research and development for zoonotic disease control. Emerg. Infect. Dis. 19 (2), 189–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dar O, Hogarth S, McIntyre S, 2013b. Tempering the risk: rift Valley fever and bioterrorism. Trop. Med. Int. Health 18 (8), 1036–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubney R, Hudson JR, 1931. Enzootic hepatitis or Rift Valley Fever: an undescribed virus disease of sheep, cattle, and man from east Africa. J. Pathol. Bacteriol. 34, 545–579. [Google Scholar]
- Daubney R, Hudson JR, 1933. Rift valley fever. East Afr. Med. J. 10, 2–19. [Google Scholar]
- Davies FG, Linthicum KJ, James AD, 1985. Rainfall and epizootic Rift valley fever. Bull. World Health Organ. 63 (5), 941–943. [PMC free article] [PubMed] [Google Scholar]
- Digoutte JP, Peters CJ, 1989. General aspects of the 1987 Rift Valley fever epidemic in Mauritania. Res. Virol. 140 (1), 27–30. [DOI] [PubMed] [Google Scholar]
- Dungu B, Lubisi BA, Ikegami T, 2018. Rift Valley fever vaccines: current and future needs. Curr. opin virol. 29, 8–15. [DOI] [PubMed] [Google Scholar]
- Easterday BC, Murphy LC, Bennett DG Jr., 1962. Experimental Rift Valley fever in lambs and sheep. Am. J. Vet. Res. 23, 1231–1240. [PubMed] [Google Scholar]
- Eddy GA, Peters CJ, Meadors G, Cole FE Jr., 1981. Rift Valley fever vaccine for humans. Contrib. Epidemiol. Biostat. 3, 124–141. [Google Scholar]
- Eisen L, Bolling BG, Blair CD, Beaty BJ, Moore CG, 2008. Mosquito species richness, composition, and abundance along habitat-climate-elevation gradients in the northern Colorado Front Range. J. Med. Entomol. 45 (4), 800–811. [DOI] [PubMed] [Google Scholar]
- El Mamy ABO, Baba MO, Barry Y, Isselmou K, Dia ML, Hampate B, Diallo MY, El Kory MOB, Diop M, Lo MM, Thiongane Y, Bengoumi M, Puech L, Plee L, Claes F, de La Rocque S, Doumbia B, 2011. Unexpected Rift valley fever outbreak, northern Mauritania. Emerg. Infect. Dis. 17 (10), 1894–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis J, Moon C, 2006. The US biological weapons program. In: Wheelis M, Rozsa L, Dando M (Eds.), Deadly Cultures: Biological Weapons since 1945. Harvard University Press, Cambridge, MA, pp. 9–46. [Google Scholar]
- Endicott S, Hagerman E, 1998. The United States and Biological Warfare: Secrets from the Early Cold War and Korea. Indiana University Press, Indianapolis, IN. [Google Scholar]
- Faye O, Ba H, Ba Y, Freire CC, Faye O, Ndiaye O, Elgady IO, Zanotto PM, Diallo M, Sall AA, 2014. Reemergence of Rift Valley Fever, Mauritania, 2010. Emerg. Infect. Dis. 20 (2), 300–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay GM, 1932. Rift valley fever or enzootic hepatitis. Transcr. R. Soc. Trop. Med. Hygeine 229–262 xxv. [Google Scholar]
- Fontenille D, Traore-Lamizana M, Zeller H, Mondo M, Diallo M, Digoutte JP, 1995. Short report: rift Valley fever in western Africa: isolations from Aedes mosquitoes during an interepizootic period. Am. J. Trop. Med. Hyg. 52 (5), 403–404. [DOI] [PubMed] [Google Scholar]
- Fontenille D, Traore-Lamizana M, Diallo M, Thonnon J, Digoutte JP, Zeller HG, 1998. New vectors of Rift Valley Fever in West Africa. Emerg. Infect. Dis. 4 (2), 289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Agriculture Organization of the United Nations, 2014. The Last Hurdles towards Rift Valley Fever Control. Report on the Ad Hoc Workshop on the Current State of Rift Valley Fever Vaccine and Diagnostics Development. Rome, Italy. 5–7 March 2014. [Google Scholar]
- Francis T, Magill TP, 1935. Rift valley fever : a report of three cases of laboratory infection and the experimental transmission of the disease to ferrets. J. Exp. Med. 62 (3), 433–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed I, 1951. Rift valley fever in man, complicated by retinal changes and loss of vision. S. Afr. Med. J. 25 (50), 930–932. [PubMed] [Google Scholar]
- Freire CC, Iamarino A, Soumare PO, Faye O, Sall AA, Zanotto PM, 2015. Reassortment and distinct evolutionary dynamics of Rift Valley Fever virus genomic segments. Sci. Rep. 5 (11353). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyumagwa RD, Ezekiel MJ, Nyaki A, Mdaki ML, Katale ZB, Moshiro C, Keyyu JD, 2011. Response to Rift valley fever in Tanzania: challenges and opportunities. Tanzan. J. Health Res. 13 (5 Suppl. l), 332–339. [DOI] [PubMed] [Google Scholar]
- Gad AM, Feinsod FM, Allam IH, Eisa M, Hassan AN, Soliman BA, el Said S, Saah AJ, 1986. A possible route for the introduction of Rift Valley fever virus into Egypt during 1977. J. Trop. Med. Hyg. 89 (5), 233–236. [PubMed] [Google Scholar]
- Gear J, De Meillon B, Measroch V, Davis DH, Harwin H, 1951. Rift Valley fever in South Africa. 2. The occurrence of human cases in the orange free state, the north-Western cape province, the western and southern transvaal. B. Field and laboratory investigation. S. Afr. Med. J. 25 (49), 908–912. [PubMed] [Google Scholar]
- Gear J, De Meillon B, Le Roux AF, Kofsky R, Innes RR, Steyn JJ, Oliff WD, Schulz KH, 1955. Rift valley fever in South Africa; a study of the 1953 outbreak in the Orange Free State, with special reference to the vectors and possible reservoir hosts. S. Afr. Med. J. 29 (22), 514–518. [PubMed] [Google Scholar]
- Grobbelaar AA, Weyer J, Leman PA, Kemp A, Paweska JT, Swanepoel R, 2011. Molecular epidemiology of Rift Valley fever virus. Emerg. Infect. Dis. 17 (12), 2270–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington DG, Lupton HW, Crabbs CL, Peters CJ, Reynolds JA, Slone TW Jr., 1980. Evaluation of a formalin-inactivated Rift Valley fever vaccine in sheep. Am. J. Vet. Res. 41 (10), 1559–1564. [PubMed] [Google Scholar]
- Harris SH, 1994. Factories of Death: Japanese Biological Warfare, 1932–45, and the American Cover-up. Routledge, New York, NY. [Google Scholar]
- Hartley DM, Rinderknecht JL, Nipp TL, Clarke NP, Snowder GD, 2011. Potential effects of Rift Valley fever in the United States. Emerg. Infect. Dis. 17 (8), e1 National Center for Foreign Animal and Zoonotic Disease Defense Advisory Group on Rift Valley Fever. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan OA, Ahlm C, Sang R, Evander M, 2011. The 2007 Rift Valley fever outbreak in Sudan. PLoS Neglected Trop. Dis. 5 (9), e1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himeidan YE, Kweka EJ, Mahgoub MM, El Rayah el A, Ouma JO, 2014. Recent outbreaks of Rift Valley Fever in east Africa and the middle East. Front Public Health 2, 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hise AG, Traylor Z, Hall NB, Sutherland LJ, Dahir S, Ermler ME, Muiruri S, Muchiri EM, Kazura JW, LaBeaud AD, King CH, Stein CM, 2015. Association of symptoms and severity of rift valley fever with genetic polymorphisms in human innate immune pathways. PLoS Neglected Trop. Dis. 9 (3), e0003584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogstraal H, Meegan JM, Khalil GM, Adham FK, 1979. The Rift Valley fever epizootic in Egypt 1977–78. 2. Ecological and entomological studies. Trans. R. Soc. Trop. Med. Hyg. 73 (6), 624–629. [DOI] [PubMed] [Google Scholar]
- International Institute for Peace and Conflict Research, 1971. The problem of chemical and biological Warfare. In: The Rise of CB Weapons, vol. I Almqvist & Wiksell, Stockholm, Sweden Institute SIPR. [Google Scholar]
- James Martin Center for Nonproliferation Studies. Chemical and Biological Weapons: Possession and Programs Past and Present 2008. [updated 3/20087/9/2015]. Available from: http://cns.miis.edu/cbw/possess.htm. [Google Scholar]
- Jansen van Vuren P, Shalekoff S, Grobbelaar AA, Archer BN, Thomas J, Tiemessen CT, Paweska JT, 2015. Serum levels of inflammatory cytokines in Rift Valley fever patients are indicative of severe disease. Virol. J. 12, 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BK, Ocheng D, Gitau LG, Gichogo A, Tukei PM, Ngindu A, Langatt A, Smith DH, Johnson KM, Kiley MP, Swanepoel R, Isaacson M, 1983. Viral haemorrhagic fever surveillance in Kenya, 1980–1981. Trop. Geogr. Med. 35 (1), 43–47. [PubMed] [Google Scholar]
- Jones K A brief history of the U.S. Army Project Fort Detrick Operation Whitecoat [cited 2016 24 Aug]. Available from: http://usarmywhitecoat.com/?page_id=7. [Google Scholar]
- Jupp PG, Kemp A, Grobbelaar A, Lema P, Burt FJ, Alahmed AM, Al Mujalli D, Al Khamees M, Swanepoel R, 2002. The 2000 epidemic of Rift Valley fever in Saudi Arabia: mosquito vector studies. Med. Vet. Entomol. 16 (3), 245–252. [DOI] [PubMed] [Google Scholar]
- Kark JD, Aynor Y, Peters CJ, 1982. A rift Valley fever vaccine trial. I. Side effects and serologic response over a six-month follow-up. Am. J. Epidemiol. 116 (5), 808–820. [DOI] [PubMed] [Google Scholar]
- Kasari TR, Carr DA, Lynn TV, Weaver JT, 2008. Evaluation of pathways for release of Rift Valley fever virus into domestic ruminant livestock, ruminant wildlife, and human populations in the continental United States. J. Am. Vet. Med. Assoc. 232 (4), 514–529. [DOI] [PubMed] [Google Scholar]
- Kitchen SF, 1934. Laboratory infections with the virus of Rift Valley fever. Am. J. Trop. Med. 14, 547–564. [Google Scholar]
- Konrad SK, Miller SN, 2012. A temperature-limited assessment of the risk of Rift Valley fever transmission and establishment in the continental United States of America. Geospatial health 6 (2), 161–170. [DOI] [PubMed] [Google Scholar]
- LaBeaud AD, Pfeil S, Muiruri S, Dahir S, Sutherland LJ, Traylor Z, Gildengorin G, Muchiri EM, Morrill J, Peters CJ, Hise AG, Kazura JW, King CH, 2015. Factors associated with severe human Rift valley fever in sangailu, Garissa county, Kenya. PLoS Neglected Trop. Dis. 9 (3), e0003548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin LW, Meegan JM, Strausbaugh LJ, Morens DM, Watten RH, 1979. Epidemic Rift Valley fever in Egypt: observations of the spectrum of human illness. Trans. R. Soc. Trop. Med. Hyg. 73 (6), 630–633. [DOI] [PubMed] [Google Scholar]
- Leitenberg M, Zilinskas RA, 2012. The Soviet Biological Weapons Program: a History. Harvard University Press, Cambridge, MA. [Google Scholar]
- Linthicum KJ, Britch SC, Anyamba A, 2016. Rift valley fever: an emerging mosquitoborne disease. Annu. Rev. Entomol. 61, 395–415. [DOI] [PubMed] [Google Scholar]
- Liu J, Sun Y, Shi W, Tan S, Pan Y, Cui S, Zhang Q, Dou X, Lv Y, Li X, Li X, Chen L, Quan C, Wang Q, Zhao Y, lv Q, Hua W, Zeng H, Chen Z, Xiong H, Jiang C, Pang X, Zhang F, Liang M, Wu G, Gao GF, Liu WJ, Li A, Wang Q, 2017. The first imported case of Rift Valley fever in China reveals a genetic reassortment of different viral lineages. Emerg. Microb. Infect. 6, e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madani TA, Al-Mazrou YY, Al-Jeffri MH, Mishkhas AA, Al-Rabeah AM, Turkistani AM, Al-Sayed MO, Abodahish AA, Khan AS, Ksiazek TG, Shobokshi O, 2003. Rift Valley fever epidemic in Saudi Arabia: epidemiological, clinical, and laboratory characteristics. Clin. Infect. Dis. 37 (8), 1084–1092. [DOI] [PubMed] [Google Scholar]
- Mandell RB, Flick R, 2011. Rift valley fever virus: a real bioterror threat. Bioterr. Biodef. 2 (2), 108. [Google Scholar]
- Max JM-M, Michael T, 2018. History of mosquitoborne diseases in the United States and implications for new pathogens. Emerg, Infect. Dis. j. 24 (5), 821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy AK, Nichol ST, 2012. Rift Valley fever virus inhibits a pro-inflammatory response in experimentally infected human monocyte derived macrophages and a pro-inflammatory cytokine response may be associated with patient survival during natural infection. Virology 422 (1), 6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh BM, 1972. Rift Valley fever. 1. Vector studies in the field. J. S. Afr. Vet. Med. Assoc. 43 (4), 391–395. [PubMed] [Google Scholar]
- McIntosh BM, Russell D, dos Santos I, Gear JH, 1980a. Rift Valley fever in humans in South Africa. S. Afr. Med. J. 58 (20), 803–806. [PubMed] [Google Scholar]
- McIntosh BM, Jupp PG, dos Santos I, Barnard BJ, 1980b. Vector studies on Rift valley fever virus in South Africa. S. Afr. Med. J. 58 (3), 127–132. [PubMed] [Google Scholar]
- Meegan JM, 1979. The Rift Valley fever epizootic in Egypt 1977–78. 1. Description of the epizootic and virological studies. Trans. R. Soc. Trop. Med. Hyg. 73 (6), 618–623. [DOI] [PubMed] [Google Scholar]
- Meegan JM, Watten RH, Laughlin LW, 1981. Clinical experience with Rift valley fever in humans during the 1977 Egyptian epizootic. Contrib. Epidemiol. Biostat. 3, 114–123. [Google Scholar]
- Miller WS, Demciiak P, Rosenberger CR, Dominik JW, Bradshaw JL, 1963. Stability and infectivity of airborne yellow fever and Rift valley fever viruses. Am. J. Hygeine 77, 114–121. [Google Scholar]
- Miller BR, Godsey MS, Crabtree MB, Savage HM, Al-Mazrao Y, Al-Jeffri MH, Abdoon AM, Al-Seghayer SM, Al-Shahrani AM, Ksiazek TG, 2002. Isolation and genetic characterization of Rift Valley fever virus from Aedes vexans arabiensis, Kingdom of Saudi Arabia. Emerg. Infect. Dis. 8 (12), 1492–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed M, Mosha F, Mghamba J, Zaki SR, Shieh WJ, Paweska J, Omulo S, Gikundi S, Mmbuji P, Bloland P, Zeidner N, Kalinga R, Breiman RF, Njenga MK, 2010. Epidemiologic and clinical aspects of a Rift Valley fever outbreak in humans in Tanzania, 2007. Am. J. Trop. Med. Hyg. 83 (2 Suppl. l), 22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery RE, 1913. Report of the Veterinary Department (R. J. STORDY, Chief Vet. Officer) for the Year 1912–13. Ann Rep Dep Agriculture, Kenya Colony. [Google Scholar]
- Morvan J, Fontenille D, Saluzzo JF, Coulanges P, 1991. Possible Rift Valley fever outbreak in man and cattle in Madagascar. Trans. R. Soc. Trop. Med. Hyg. 85 (1), 108. [DOI] [PubMed] [Google Scholar]
- Morvan J, Rollin PE, Laventure S, Rakotoarivony I, Roux J, 1992. Rift Valley fever epizootic in the central highlands of Madagascar. Res. Virol. 143 (6), 407–415. [DOI] [PubMed] [Google Scholar]
- Murithi RM, Munyua P, Ithondeka PM, Macharia JM, Hightower A, Luman ET, Breiman RF, Njenga MK, 2011. Rift Valley fever in Kenya: history of epizootics and identification of vulnerable districts. Epidemiol. Infect. 139 (3), 372–380. [DOI] [PubMed] [Google Scholar]
- Nabeth P, Kane Y, Abdalahi MO, Diallo M, Ndiaye K, Ba K, Schneegans F, Sall AA, Mathiot C, 2001. Rift Valley fever outbreak, Mauritania, 1998: seroepidemiologic, virologic, entomologic, and zoologic investigations. Emerg. Infect. Dis. 7 (6), 1052–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasci RS, Moore CG, 1998. Vector-borne disease surveillance and natural disasters. Emerg. Infect. Dis. 4 (2), 333–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndiva Mongoh M, Hearne R, Dyer NW, Khaitsa ML, 2008. The economic impact of West Nile virus infection in horses in the North Dakota equine industry in 2002. Trop. Anim. Health Prod. 40 (1), 69–76. [DOI] [PubMed] [Google Scholar]
- Niklasson B, 1982. Rift Valley fever virus vaccine trial: study of side-effects in humans. Scand. J. Infect. Dis. 14 (2), 105–109. [DOI] [PubMed] [Google Scholar]
- Niklasson B, Liljestrand J, Bergstrom S, Peters CJ, 1987. Rift Valley fever: a seroepidemiological survey among pregnant women in Mozambique. Epidemiol. Infect. 99 (2), 517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters CJ, 1997. Virus Hunter: Thirty Years of Battling Hot Viruses Around the World. Random House, New York, NY. [Google Scholar]
- Pienaar NJ, Thompson PN, 2013. Temporal and spatial history of Rift Valley fever in South Africa: 1950 to 2011. Onderstepoort J. Vet. Res. 80 (1), 384. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Fang Y, Martinez VM, 2006. Effects of temperature on the transmission of west nile virus by Culex tarsalis (Diptera: Culicidae). J. Med. Entomol. 43 (2), 309–317. [DOI] [PubMed] [Google Scholar]
- Rolin AI, Berrang-Ford L, Kulkarni MA, 2013. The risk of Rift Valley fever virus introduction and establishment in the United States and European Union. Emerg. Microb. Infect. 2 (12), e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabin AB, Blumberg RW, 1947. Human infection with Rift Valley fever virus and immunity twelve years after single attack. Proc Soc Exp Biol Med 64 (4), 385–389. [DOI] [PubMed] [Google Scholar]
- Sall AA, Zanotto PM, Sene OK, Zeller HG, Digoutte JP, Thiongane Y, Bouloy M, 1999. Genetic reassortment of Rift Valley fever virus in nature. J. Virol. 73 (10), 8196–8200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saluzzo JF, Digoutte JP, Chartier C, Martinez D, Bada R, 1987. Focus of Rift Valley fever virus transmission in southern Mauritania. Lancet 1 (8531), 504. [DOI] [PubMed] [Google Scholar]
- Samy AM, Peterson AT, Hall M, 2017a. Phylogeography of Rift Valley fever virus in Africa and the arabian peninsula. PLoS Neglected Trop. Dis. 11 (1), e0005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samy AM, Peterson AT, Hall M, 2017b. Phylogeography of Rift Valley fever virus in Africa and the arabian peninsula. PLoS Neglected Trop. Dis. 11 (1), e0005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwentker FF, Rivers TM, 1934. Rift valley fever in man : report of a fatal laboratory infection complicated by thrombophlebitis. J. Exp. Med. 59 (3), 305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh WJ, Paddock CD, Lederman E, Rao CY, Gould LH, Mohamed M, Mosha F, Mghamba J, Bloland P, Njenga MK, Mutonga D, Samuel AA, Guarner J, Breiman RF, Zaki SR, 2010. Pathologic studies on suspect animal and human cases of Rift Valley fever from an outbreak in Eastern Africa, 2006–2007. Am. J. Trop. Med. Hyg. 83 (2 Suppl. l), 38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker T, Boulianne C, Vincent MJ, Pezzanite L, Al-Qahtani MM, Al-Mazrou Y, Khan AS, Rollin PE, Swanepoel R, Ksiazek TG, Nichol ST, 2002. Genetic analysis of viruses associated with emergence of Rift Valley fever in Saudi Arabia and Yemen, 2000–01. Emerg. Infect. Dis. 8 (12), 1415–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siam AL, Meegan JM, Gharbawi KF, 1980. Rift Valley fever ocular manifestations: observations during the 1977 epidemic in Egypt. Br. J. Ophthalmol. 64 (5), 366–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindato C, Pfeiffer DU, Karimuribo ED, Mboera LE, Rweyemamu MM, Paweska JT, 2015. A spatial analysis of Rift Valley fever virus seropositivity in domestic ruminants in Tanzania. PLoS One 10 (7), e0131873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithburn KC, Haddow AJ, Gillett JD, 1948. Rift Valley fever; isolation of the virus from wild mosquitoes. Br. J. Exp. Pathol. 29 (2), 107–121. [PMC free article] [PubMed] [Google Scholar]
- Smithburn KC, Haddow AJ, Lumsden WH, 1949a. Rift Valley fever; transmission of the virus by mosquitoes. Br. J. Exp. Pathol. 30 (1), 35–47. [PMC free article] [PubMed] [Google Scholar]
- Smithburn KC, Mahaffy AF, Haddow AJ, Kitchen SF, Smith JF, 1949b. Rift Valley fever; accidental infections among laboratory workers. J. Immunol. 62 (2), 213–227. [PubMed] [Google Scholar]
- Sow A, Faye O, Ba Y, Ba H, Diallo D, Loucoubar C, Boushab M, Barry Y, Diallo M, Sall AA, 2014. Rift Valley fever outbreak, southern Mauritania, 2012. Emerg. Infect. Dis. 20 (2), 296–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee on Special Immunizations Program for Laboratory Personnel Engaged in Research on Countermeasures for Select Agents, 2011. Protecting the Frontline in Biodefense Research: the Special Immunizations Program. National Academies Press, Washington, DC. [PubMed] [Google Scholar]
- Turell MJ, Perkins PV, 1990. Transmission of Rift Valley fever virus by the sand fly, Phlebotomus duboscqi (Diptera: psychodidae). Am. J. Trop. Med. Hyg. 42 (2), 185–188. [DOI] [PubMed] [Google Scholar]
- Turell MJ, Presley SM, Gad AM, Cope SE, Dohm DJ, Morrill JC, Arthur RR, 1996. Vector competence of Egyptian mosquitoes for Rift Valley fever virus. Am. J. Trop. Med. Hyg. 54 (2), 136–139. [DOI] [PubMed] [Google Scholar]
- Turell MJ, Linthicum KJ, Patrican LA, Davies FG, Kairo A, Bailey CL, 2008. Vector competence of selected African mosquito (Diptera: Culicidae) species for Rift Valley fever virus. J. Med. Entomol. 45 (1), 102–108. [DOI] [PubMed] [Google Scholar]
- Turell MJ, Britch SC, Aldridge RL, Kline DL, Boohene C, Linthicum KJ, 2013. Potential for mosquitoes (Diptera: Culicidae) from Florida to transmit Rift valley fever virus. J. Med. Entomol. 50 (5), 1111–1117. [DOI] [PubMed] [Google Scholar]
- Turkistany AH, Mohamed AG, Al-Hamdan N, 2001. Seroprevalence of Rift Valley fever among slaughterhouse personnel in makkah during Hajj 1419h (1999). J family community med. 8 (3), 53–57. [PMC free article] [PubMed] [Google Scholar]
- van Velden DJ, Meyer JD, Olivier J, Gear JH, McIntosh B, 1977. Rift Valley fever affecting humans in South Africa: a clinicopathological study. S. Afr. Med. J. 51 (24), 867–871. [PubMed] [Google Scholar]
- Vloet RPM, Vogels CBF, Koenraadt CJM, Pijlman GP, Eiden M, Gonzales JL, van Keulen LJM, Wichgers Schreur PJ, Kortekaas J, 2017. Transmission of Rift Valley fever virus from European-breed lambs to Culex pipiens mosquitoes. PLoS Neglected Trop. Dis. 11 (12), e0006145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichgers Schreur PJ, van Keulen L, Kant J, Oreshkova N, Moormann RJ, Kortekaas J, 2016. Co-housing of Rift Valley fever virus infected lambs with immunocompetent or immunosuppressed lambs does not result in virus transmission. Front. Microbiol. 7, 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, 2015. Workshop on Prioritization of Pathogens. December 8-9, 2015. URL. http://www.who.int/blueprint/what/research-development/meeting-report-prioritization.pdf?ua=1.
- World Health Organization, 2016. Rift Valley Fever in Niger. [updated November 24, 2016]. http://www.who.int/csr/don/24-november-2016-rift-valley-fever-niger/en/.
- World Health Organization, 2017. Annual review of diseases prioritized under the research and development blueprint. In: Workshop on Prioritization of Pathogens. January 24-25, 2017, http://www.who.int/blueprint/what/research-development/2017-Prioritization-Long-Report.pdf?ua=1.
- World Health Organization, 2018a. Rift Valley Fever – Gambia. [cited 2018 3/16/18]. Available from: http://www.who.int/csr/don/26-february-2018-rift-valley-fever-gambia/en/.
- World Health Organization. South Sudan declares Rift Valley fever outbreak in parts of Eastern Lakes State 2018. [updated March 12, 2018; cited 2018 3/16/18]. Available from: http://www.afro.who.int/news/south-sudan-declares-rift-valley-fever-outbreak-parts-eastern-lakes-state.
- World Health Organization. Rift Valley Fever fact sheet. [February/19/2018]. http://www.who.int/mediacentre/factsheets/fs207/en/.
- World Organization for Animal Health (OIE). OIE-Listed diseases, infections and infestations in force in 2018 Available from: http://www.oie.int/en/animal-health-in-the-world/oie-listed-diseases-2018/.
- Yedloutschnig RJ, Dardiri AH, Walker JS, 1981. Rift Valley Fever infection in sheep by contact exposure. Contrib. Epidemiol. Biostat. 3, 53–59. [Google Scholar]
- Zeller HG, Fontenille D, Traore-Lamizana M, Thiongane Y, Digoutte JP, 1997. Enzootic activity of Rift Valley fever virus in Senegal. Am. J. Trop. Med. Hyg. 56 (3), 265–272. [DOI] [PubMed] [Google Scholar]


