Abstract
Integrins are heterodimeric transmembrane receptors composed of α and β chains, with an N-terminal extracellular domain forming a globular head corresponding to the ligand binding site. Integrins regulate various cellular functions including adhesion, migration, proliferation, spreading and apoptosis. On platelets, integrins play a central role in adhesion and aggregation on subendothelial matrix proteins of the vascular wall, thereby ensuring hemostasis. Platelet integrins belong either to the β1 family (α2β1, α5β1 and α6β1) or to the β3 family (αIIbβ3 and αvβ3). On resting platelets, integrins can engage their ligands when the latter are immobilized but not in their soluble form. The effects of various agonists promote an inside-out signal in platelets, increasing the affinity of integrins for their ligands and conveying a modest signal reinforcing platelet activation, called outside-in signaling. This outside-in signal ensures platelet adhesion, shape change, granule secretion and aggregation. In this review, we examine the role of each platelet integrin in hemostatic plug formation, hemostasis and arterial thrombosis and also beyond these classical functions, notably in tumor metastasis and sepsis.
Blood platelets: their role in hemostasis, arterial thrombosis and beyond
Blood platelets are small, anucleate, discoid cells that are derived from cytoplasmic extensions of megakaryocytes into the bone marrow sinusoids.1 Platelets play a major role in hemostasis by arresting bleeding following vascular injury. They adhere, become activated and aggregate at the lesion site to form a hemostatic plug that reduces the bleeding, a process called primary hemostasis. A similar process can occur under pathological conditions, following rupture or injury of an evolved atherosclerotic plaque in a diseased artery. Platelets adhere, activate and aggregate on the exposed reactive plaque material. The resulting thrombus can cause vessel occlusion at the lesion site, or form emboli which can occlude a downstream vessel and lead to severe ischemic pathologies such as myocardial infarction, ischemic stroke and obstructive peripheral arterial disease.2 Platelets also maintain vascular integrity as evidenced by endothelial thinning and fenes-tration in the capillaries and post-capillary venules of thrombocytopenic rabbits.3 This was confirmed by the leakage of blood fluids from vessels of thrombocytopenic mice.4 Furthermore, platelets prevent inflammatory bleeding as evidenced by local bleeding under inflammatory conditions in the skin, lungs, and brain of thrombo-cytopenic mice.5
The molecular mechanisms of platelet interactions with the vascular wall are well documented. Under high shear conditions, von Willebrand factor (vWF), present in the subendothelium and adsorbed from plasma onto adhesive proteins of the vessel wall, recruits platelets through its interaction with the glycoprotein (GP) Ib-IX-V complex. Stable adhesion of platelets is then ensured by the β1 integrin family, namely α2β1, α5β1 and α6β1, which interact with collagen, fibronectin and laminins, respectively. Integrin αIIbβ3 is also implicated in stable platelet adhesion, mostly through its interactions with vWF and subendothe-lial fibronectin. The capture of platelets facilitates the interaction of GPVI with collagen, inducing an intracellular signaling cascade leading to sustained platelet activa-tion.6,7 The latter results in platelet shape change, filopodia formation and secretion of the contents of intracellular granules, including adenosine diphosphate (ADP) and ade-nosine triphosphate. Activated platelets also synthesize and liberate thromboxane A2 (TxA2). By interacting with their respective receptors, P2Y1 and P2Y12 for ADP, P2X1 for adenosine triphosphate and thromboxane and prostag-landin receptor for TxA2, these soluble agonists amplify the platelet activation, upregulating the affinity of αIIbβ3 for its main ligand, soluble fibrinogen. Fibrinogen forms bridges between adjacent platelets, ensuring platelet aggregation and formation of the hemostatic plug.8 Some activated platelets expose negatively charged phospholi-pids, phosphatidylserine, allowing the recruitment of coagulation factors, thrombin generation and the formation of an insoluble fibrin network which stabilizes the platelet plug.
Besides their role in hemostasis and thrombosis, platelets are also implicated in non-hemostatic functions. On the one hand, these functions can be physiological such as embryonic and fetal development with the involvement of C-type lectin-like receptor 2 (CLEC-2) in blood and lymphatic vessel separation,9 angiogenesis and tissue repair through their ability to release proangiogenic factors such as vascular endothelial growth factor and platelet-derived growth factor, and innate immunity through the expression of toll-like receptors 1-9 which recognize pathogens and induce secretion of antimicrobial factors.10 On the other hand, platelets are also involved in pathological processes. A role in tumor metastasis has been demonstrated by Gasic and collaborators since thrombocytope-nia reduces the capacity of tumor cells to colonize the lungs after their intravenous injection, a process reversed by platelet transfusion.11 Conversely, thrombocytopenia increases septic mortality in patients.12 Platelets have also been proposed to contribute to rheumatoid arthritis13 and autoimmune diseases such as systemic lupus erythema-
The structure and function of integrins
Integrins are a superfamily of heterodimeric transmem-brane receptors resulting from the association of two gly-coprotein chains, α and β. In man, various combinations of 18 α subunits and eight β subunits can form 24 different integrins.15 The N-terminal extracellular domain of the α subunit consists of the β propeller, thigh, and calf-1 and -2 domains. A particularity exists for nine of the integrin α chains which present over the β propeller an αI-domain containing the ligand binding site,15 a metal ion-dependent adhesion site (MIDAS).16 The N-terminal extracellular domain of the β subunit is composed of the βI, hybrid, ple-xin-semaphorin-integrin, integrin epidermal growth factor-1 to 4 and β-tail domains. The βI domain contains three metal ions site: the MIDAS, the synergistic metal binding site, also known as ligand-associated metal binding site (LIMBS), and the adjacent to MIDAS (ADMIDAS).16
The N-terminal α subunit β propeller domain (for integrins that do not present αI-domains) and the β subunit βI domain, assemble to form a globular head corresponding to the ligand binding site.15 The transmembrane domain is composed of two parallel helices in close proximity, which need to be separated for the integrin to signal.17 Finally, the short C-terminal intracellular domains of both subunits interact non-covalently and are important for integrin signaling.
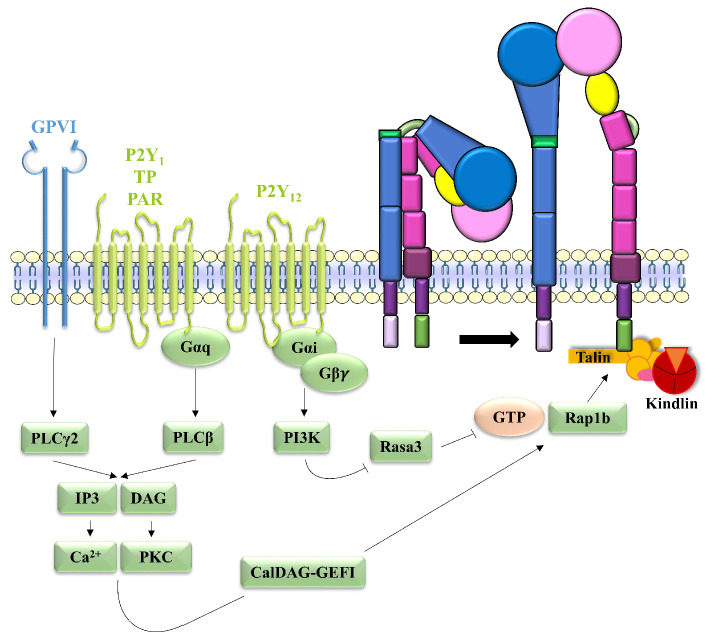
Agonist binding to platelet receptors promotes an intra-cellular signal, called inside-out signaling, which leads to a change in the conformation of the integrin extracellular domain, increasing the affinity for its ligands. Electron microscopy has enabled identification of three different integrin conformations:18 (i) in the resting state the ecto-domain is closed and folded, forming an inverted V, and the integrin has a low affinity for extracellular ligands, the binding site being close to the membrane surface; (ii) in the intermediate state the ectodomain expands, but the globular head remains closed and the integrin has an intermediate affinity for its ligands; (iii) in the high affinity state the integrin is expanded and the globular head is open, exposing the ligand binding site (Figure 1). Besides their conformational changes following cell stimulation, integrins also cluster into oligomers to increase the avidity for their ligands.19
Integrins are receptors for soluble ligands, cell surface ligands and matrix proteins which mediate cell-cell and cell-extracellular matrix interactions. They regulate general functions such as cell adhesion, migration, proliferation, spreading and apoptosis and also participate in numerous pathophysiological processes such as hemo-stasis, thrombosis and immune responses.
The repertoire of platelet integrins
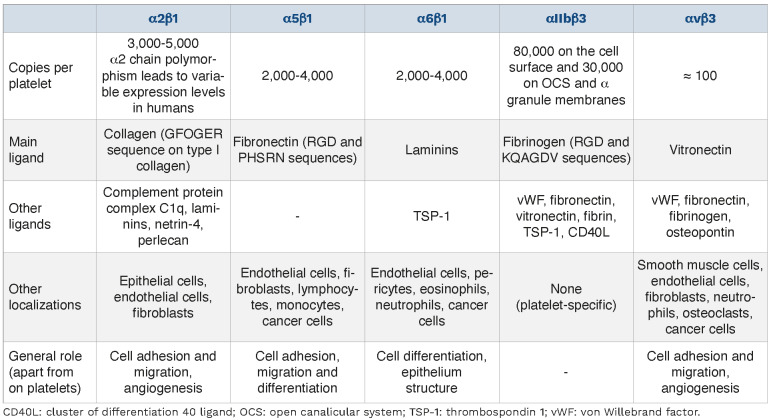
Two integrin families are expressed on platelets. Firstly, the β1 integrins α2β1, α5β1 and α6β1 which mainly ensure platelet adhesion on extracellular matrix proteins.20-22 Secondly, the β3 integrins with αIIbβ3 implicated mostly in platelet aggregation and αvβ3 for which no major hemo-static function has been identified. The expression levels, ligands, expression on other cell types and general roles of these platelet integrins are summarized in Table 1.
The role of integrins in megakaryo-cyte biology and platelet generation
The role of integrins in the regulation of megakaryopoïesis was recently described in detail in a review by Katya Ravid’s group.23 Integrins, being major adhesion receptors, have been shown to contribute to the anchorage of megakaryocytes to the extracellular matrix proteins of the bone marrow such as fibrinogen and fibronectin, notably through αIIbβ3 and α5β1, to regulate megakaryopoïesis.24,25 Most of the integrins expressed by megakaryocytes also regulate post-adhesion events such as spreading and migration, which is the case of α2β1 and αIIbβ3.26,27 While platelets are limited to the expression of six integrins, it has been proposed that megakaryocytes express two additional ones, namely α3β1 and α4β1.28,29 Integrin α3β1 has been reported to mediate the interaction of megakaryocytes with a fibroblast matrix and reduced fibroblast proliferation, suggesting a potential contribution to myelofibrosis.29 The expression of α4β1 appears limited to the early stages of megakaryocyte maturation and could contribute to this process.28,30
Figure 1.
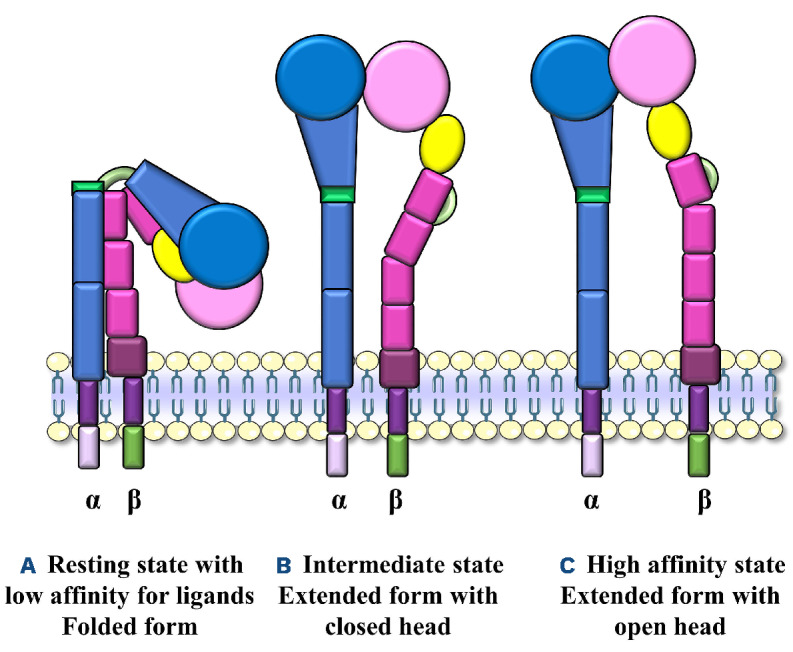
Integrin conformations. (A) Integrin in the resting state, with a folded ectodomain and low affinity for its ligands. (B) Integrin in the intermediate state with an extended ectodomain and a closed globular head. It has an intermediate affinity for its ligands. (C) Integrin in the high affinity state for its ligands, with an extended ectodomain and opened globular head exposing the ligand binding site.
Table 1.
Expression levels, ligands, expression on other cell types and general roles of platelet integrins.
With regard to platelet generation, it has been reported that only platelet-expressed integrins on megakaryocytes, αvβ3, αIIbβ3, α2β1, α5β1 and α6β1, contribute to a distinct degree to proplatelet extension or regulation.23,27,31-33 It should however be noted that α2-, α5- and α6-deficient mice have a normal platelet count, while α3 knockouts have only a modest reduction, which does not suggest a key role of these integrins on their own in platelet pro-duction.34-36 In agreement, patients with Glanzmann thrombasthenia have a platelet count in the normal range, but patients with a gain-of-function mutation exhibit macrothrombocytopenia, suggesting a potential role of αIIbβ3 in the regulation of platelet count.37 In addition, it is possible that integrins compensate for each other, as they do for platelet function, and that multiple deficiencies in mice could highlight a more prominent role in platelet production.
The activation state of integrins and integrin signaling
Integrins constantly oscillate between a low and a high affinity state, and it is well accepted that on resting platelets, integrins are mainly present in a low affinity state. Activation of platelets through many different receptors generates inside-out signaling, resulting in upregulation of the affinity of integrins which change to a high affinity state and become able to bind adhesive proteins in their soluble form, which is best illustrated by αIIbβ3 becoming able to bind fibrinogen in solution. However, when ligands (vWF, fibrinogen, laminins, fibronectin, collagen…) are immobilized on a surface, integrins of resting platelets can engage and support recruitment of resting, unstimulated platelets.38,39 This has been demonstrated in numerous flow-based experiments in which whole blood perfused over immobilized adhesive proteins showed efficient platelet adhesion through either β1 or β3 integrins. It should however be underlined that integrins ensure platelet capture only under low or intermediate flow (<900 s-1), as above such a threshold, GPIb is essential.40 This is most likely explained by the fact that immobilized adhesive proteins present a different conformation from that in solution, exposing cryptic sites and increasing the affinity of integrins. This has been well described for vWF which is in a globular form in solution and unfolds even under low shear when immobilized on a surface.41 This observation also indicates that platelet integrins do not require efficient inside-out signals to engage immobilized ligands, suggesting that platelets contain a sufficient number of integrins in a ready-to-go state to ensure rapid adhesion. Nevertheless, this is not incompatible with the fact that platelet activation certainly increases the affinity of an elevated number of integrins for their ligands. In the following paragraphs, two distinct aspects of integrin signaling will be discussed, one called inside-out signaling which leads to integrin activation and is mediated by binding of agonists to platelet receptors, and the other conveyed by the integrin, which ensures platelet adhesion and induces outside-in signaling, reinforcing platelet shape change, granule secretion and aggregation.
Activation of platelet integrins (inside-out signaling)
The binding of agonists such as collagen, ADP, TxA2 and thrombin to their respective platelet receptors, GPVI, P2Y1 and P2Y, thromboxane and prostaglandin receptor and12 protease-activated receptor, promotes integrin activation through inside-out signaling, which involves the activation of phospholipase C (PLC) |3 ory pathways. PLC hydrolyzes phosphatidylinositol (4,5)-bisphosphate into diacylglycerol and inositol-1,4,5-triphosphate (IP3), thereby activating protein kinase C (PKC) and mobilizing intracellular calcium42 through IP3 receptor channels. The post-calcium events of inside-out signaling have been particularly well-described for aIIb|33 activation. PKC and intracellular calcium, via calcium and diacylglycerol-regulated guanine nucleotide exchange factor I binding and activation, induce the conversion of Ras-related protein (Rap) 1b-GDP into Rap1b-GTP, the activated form.43 Activated Rap1b is then translocated to the plasma membrane and binds to talin, which interacts with the |33 cytoplasmic tail to change the conformation of the integrin and induce its activation.44 This was demonstrated using mice with a |33 L746A substitution selectively disrupting the interaction between |33 and talin and mice with a defect in talin-1; aIIb|33 activation was reduced in these animals, resulting in defective platelet aggregation and an increased bleeding time.45,46 Kindlin-3 was also shown to participate in |33 integrin activation by enhancing the interaction between talin and the |33 subunit.47 Other proteins are involved in the last step of |33 integrin activation, such as integrin-linked kinase, which serves as an adaptor protein forming an integrin-linked kinase/PINCH/parvin complex linked to the |33 tail,48 adhesion and degranulation promoting adapter protein and paxillin, which act as bridging molecules between talin and kindlin.49,50 Moreover, there are also negative regulators of the activation of platelet |33 in-tegrin, such as Ras GTPase-activating protein 3 (Rasa3). On resting platelets, Rasa3 in close proximity to integrins in the plasma membrane has a negative regulatory effect. The mechanism by which activated phosphoinositide 3-kinase (PI3K) impairs Rasa3 activity, thus inducing integrin activation, is still unknown51 (Figure 2). Regulators of G-protein signaling (RGS) also act as negative regulators since platelets from Rgs10-/- and Rgs16-/- mice present increased integrin activation, as measured by flow cytometry.52,53 Other proteins, such as a-actinin54 and calcium and integrin-binding protein 1,55 would also be involved in this negative regulation through a competitive effect at the talin binding site.
Figure 2.
Mechanism of aIIbβ3 inside-out signaling. On platelets, the collagen-GPVI interaction induces PLCy2 activation while ADP-P2Y1, TxA2-TP and thrombin-PAR interactions induce PLCβ activation. Activated PLC then generates IP3 and DAG, which mobilize intracellular calcium and activate PKC, respectively, leading to CalDAG-GEFI activation. The ADP-P2Y12 interaction activates PI3K which inhibits Rasa3. Activated CalDAG-GEFI and inhibited Rasa3 induce Rap1b activation, which binds to talin, thereby enabling activation of integrin αIIb(33 and a change in its conformation. Kindlin-3, by enhancing the interaction between talin and αIIb(33, also participates in αIIb(33 activation. ADP: adenosine diphosphate; ATP: adenosine triphosphate; CalDAG-GEFI: calcium and diacylglycerol-regulated guanine nucleotide exchange factor I; DAG: diacylglycerol; GPVI: glycoprotein VI; GTP: guanosine triphosphate; IP3: inositol-1,4,5-triphosphate; PAR: protease-activated receptor; PI3K: phosphoinositide 3-kinase; PKC: protein kinase C; PLC: phospholipase C; RAP1b: Ras-related protein 1b; Rasa3: Ras GTPase-activating protein 3; TP: thromboxane and prostaglandin receptor; TxA2: thromboxane A2.
The molecular mechanisms of β1 integrin activation are less well known. It has been proposed that the mechanism of activation of α2β1 might resemble that of αIIbβ3, with a rise in intracytoplasmic calcium levels and the involvement of talin and kindlin-3.47,56 It has been suggested that PI3K,57 actin polymerization and Rho GTPase58 may also be implicated. In contrast to αIIbβ3 and α2β1, in α5β1 inside-out signaling, kindlin-1 and -2 would have an inhibitory rather than an activating effect.59
Platelet outside-in signaling
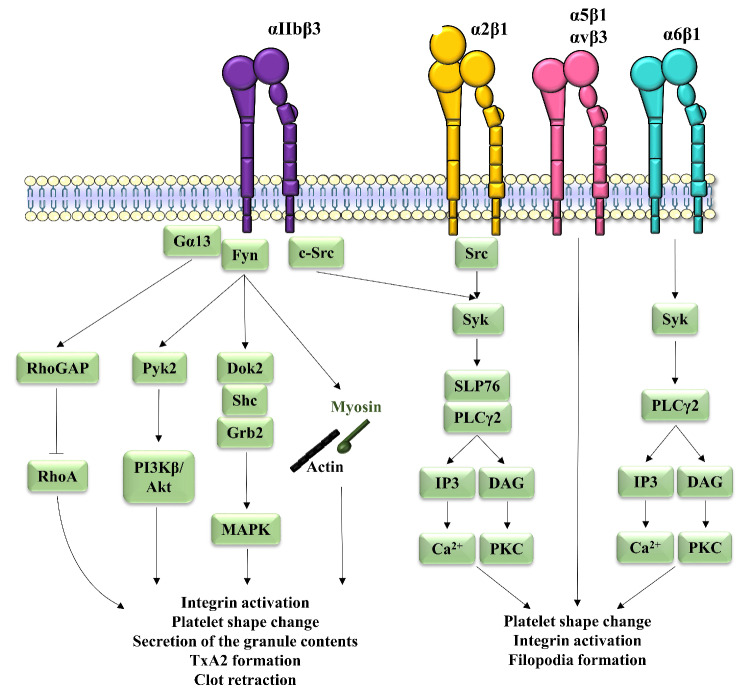
Once ligands have bound to platelet β1 or β3 integrins, they induce a signaling cascade called outside-in signaling. For α2β1 and αIIbβ3, this outside-in signaling involves Src kinase which is constitutively associated to the |3 subunit and phosphorylated on tyrosine 529 to maintain its inhibition.60 The integrin-ligand interaction induces protein-tyrosine phosphatase-1B recruitment, dephosphorylation of Src and subsequent Src activation.61 Activated Src recruits and phosphorylates spleen tyrosine kinase (Syk), which is then in an activated state.60 Activated Syk phosphorylates SLP76 and PLCy2 to induce the formation of a signaling complex,62-64 including PI3K in the case of αIIb|33.65 Activated PLCy2 then generates IP3 and diacylglycerol, leading to intracellular calcium mobilization and activation of PKC, respectively. Together, these events result in further integrin activation, platelet shape change and filopodia formation. With regard to platelet α6|31, only the implication of Syk and PLCy2 has been described,66 while the outside-in signaling cascades of α5|31 and αv|33 have not yet been studied (Figure 3).
Apart from the best known actors involved in outside-in signaling downstream of αIIbβ3, additional players have been proposed. The auto-phosphorylation of Fyn, another Src family kinase linked to the β3 cytoplasmic tail, has been shown to promote the phosphorylation of two tyro-sines of the β3 tail, Tyr747 and Tyr759,67 inducing: (i) the phosphorylation and activation of proline-rich tyrosine ki-nase 2 which stimulates PI3Kβ and activates the Akt pathway to regulate platelet adhesion and spreading;68 (ii) adaptor protein phosphorylation in the form of Dok2,69 Grb2 and Shc associated with β3,70 leading to activation of the mitogen-activated protein kinase pathway; and (iii) recruitment of myosin linked to the β3 cytoplasmic tail and interaction with the actin cytoskeleton which in turn regulates platelet morphological changes.71 Moreover, c-Src and Gα13 cluster with the β3 tail, inducing Rho GTPase-activating protein, which inhibits a small GTPase from the Rho family called Ras homolog family member A, thereby causing platelet spreading. Cleavage of the link between Src and the β3 cytoplasmic tail by calpain then induces Ras homolog family member A activation and clot retraction.72 These pathways enhance αIIb|33 activation and promote platelet shape change, granule content release and TxA2 synthesis as well as fibrin clot retraction (Figure 3). In addition, it has been suggested that αIIb|33 could use FcyRIIA as a signaling partner. The interaction of αIIb|33 with its ligand would induce intracellular phosphorylation of FcyRIIA and amplify the platelet activation signal.73,74 However, this concept was recently challenged by work from our group which did not identify a major functional role of FcyRIIA in the outside-in signaling of αIIb|33.75
Figure 3.
Mechanisms of platelet integrin outside-in signaling. Binding of fibrinogen to αIIb|33 induces c-Src activation followed by the recruitment of Syk, which phosphorylates SLP76 and PLCy2, leading to the formation of a signaling complex. Activated PLCy2 then generates IP3 and DAG, which mobilize intracellular calcium and activate PKC, respectively. Additional factors have been proposed to participate in the outside-in signaling of αIIb|33 with the auto-phosphorylation of Fyn enabling the phosphorylation of tyrosines on (33, thereby inducing: (i) activation of Pyk2 and stimulation of PI3K|3 and Akt; (ii) Dok2, Grb2 and Shc phosphorylation leading to MAPK activation; (iii) recruitment of myosin linked to the (33 cytoplasmic tail and interaction with the actin cytoskeleton. Moreover, the clustering of c-Src and Gα13 induces RhoGAP activation, inhibiting RhoA. Altogether, these events promote further integrin activation, platelet shape change, granule content release and TxA2 synthesis as well as clot retraction. The α2|31-collagen interaction induces outside-in signaling through Src activation leading to recruitment of Syk, which phosphorylates SLP76 and PLCy2. Similarly, the α6|31-laminin interaction induces outside-in signaling through Syk recruitment followed by PLCy2 phosphorylation. PLCy2 generates IP3 and DAG, which mobilize intracellular calcium and activate PKC, respectively. These outside-in signaling pathways lead to platelet shape change, integrin activation and filopodia formation. The outside-in signaling mechanisms of α5|31 and αv(33 have not yet been studied. DAG: diacylglycerol; Dok2: docking protein 2; Grb2: growth factor receptor-bound protein 2; IP3: inositol-1,4,5-triphosphate; MAPK: mitogen-activated protein kinase; PI3K: phosphoinositide 3-kinase; PKC: protein kinase C; PLC: phospholipase C; Pyk2: proline-rich tyrosine kinase 2; RhoA: Ras homolog family member A; RhoGAP: Rho GTPase-activating protein; Syk: spleen tyrosine kinase; TxA2: thromboxane A2.
Role of GPVI in platelet activation on immobilized fibrinogen: impact on the importance of αIIb(33 outside-in signaling
We recently identified a major role of GPVI in platelet activation on fibrinogen, which is instrumental in platelet spreading, but not adhesion.76,77 This activation occurs via a direct interaction between GPVI and fibrinogen, while αIIb|33 is required to support platelet adhesion. This finding indicates that GPVI mediates a signal following platelet adhesion to fibrinogen which is distinct from αIIb|33 outside-in signaling and that the signaling measured upon platelet adhesion to fibrinogen cannot be exclusively related to outside-in signaling as reported so far. Importantly, only human but not murine GPVI is a functional receptor for fibrinogen,78 which explains a long unanswered question why resting mouse platelets deposited on immobilized fibrinogen do not spread. This was notably demonstrated by the observation that expression of human GPVI in mouse platelets led to a nice spreading on fibrinogen as human platelets do.77 We proposed that the GPVI-fibrinogen interaction is functionally important as it results in thrombus build-up. As a consequence, these observations indicate that the outside-in signaling of αIIb|33, which has been assessed in a model consisting of depositing platelets on immobilized fibrinogen, has been overestimated and that some of the actors identified might not even be specific as they could belong to the GPVI signaling pathway. A re-investigation of αIIb|33 outside-in signaling, in the absence of GPVI, appears required for a more thorough investigation of the signaling actors really involved downstream of platelet integrins and their physiological importance.
The role of integrins in the formation of a hemostatic plug
Integrins play a major role in platelet adhesion at the site of vascular injury. Under low shear flow (<900 s-1), integrins are sufficient to allow the capture of platelets on their immobilized ligands, while at higher shear (>900 s-1), the GPIb-IX complex is required to recruit platelets to vWF immobilized on adhesive proteins, with integrins ensuring stable adhesion.40 In mice, the threshold shear rate needed for integrins to capture circulating platelets is much higher than in humans.40,79 Platelet integrins contribute to platelet activation through outside-in signaling, but the level of activation generated is very modest compared to that induced by soluble agonists.36,66,80 Concerning aggregation, αIIbβ3 is the major platelet integrin involved due to its ability to bind plasma fibrinogen, thereby enabling platelet-platelet interactions. αIIbβ3-vWF bonds are also implicated in platelet aggregate formation; this was found to be important in the absence or presence of low levels of fibrinogen, notably in aggregates formed in blood from afibrinogenemia patients.81 However, vWF also supports platelet aggregation in the presence of fibrinogen since: (i) flowing platelets attach to vWF under a very wide range of wall shear rates, including low shears of 100 s-1;40 and (ii) vWF on activated platelets is central to enabling platelet attachment to thrombi, indicating the key role of GPIb-vWF bonds in platelet aggregation under normal conditions, i.e. in the presence of fibrinogen.82 Furthermore, under very high shear conditions, platelet aggregation depends strongly on vWF through its ability to unfold, bind platelets and form rolling aggregates.83 In addition to β3, platelet α5β1 could be implicated in thrombus formation in vitro on collagen. Indeed, perfusion of whole blood from mice genetically deficient in α5 in the platelet linage (PF4Cre-α5-/-) over collagen, a surface that does not activate α5β1, resulted in a reduced thrombus volume as compared to that following perfusion of whole blood from control mice.35 This suggests that α5β1 contributes to thrombus formation through an interaction with plasma fibronectin. However, the same effect was not observed using human blood perfused over collagen in the presence of an anti-α5 blocking antibody.
The importance of platelet integrins in hemostasis
Despite their involvement in platelet adhesion and aggregation, platelet β1 integrins do not seem to play a crucial role in hemostasis since the only patient described to have an α2β1 defect was a female who displayed moderate hemorrhages limited to childhood, which disappeared after puberty.84 Furthermore, α6β1-deficient patients do not have a bleeding diathesis and no patients with α5β1 deficiency have been reported. These observations were confirmed in mice in which inactivation of α2,34,85 α535 or α6 genes36 did not modify the tail bleeding time. Moreover, mice deficient in all three β1 integrins in the platelet linage (PF4Cre-β1-/-) had a normal bleeding time,86 in agreement with results obtained in our laboratory (Online Supplementary Figure S1A, B). Nevertheless, another mouse strain deficient in all three β1 integrins on hematopoietic cells did have an increased tail bleeding time,87 suggesting compensatory mechanisms between the different β1 integrins. The variation in bleeding time between these mouse strains is difficult to explain, but could arise from the different targeting approaches, i.e., a deficiency in the platelet linage versus all hematopoietic cells.
Integrin αIIbβ3 is well known to play a critical role in hemostasis, as illustrated by a rare and severe hemor-rhagic disease called Glanzmann thrombasthenia, which occurs when one subunit of the integrin is absent or nonfunctional. This disease is characterized by a reduction in the ability of platelets to adhere and spread on fibrinogen and to aggregate. Patients with Glanzmann thrombasthe-nia exhibit a marked hemorrhagic phenotype.88 Furthermore, mice with αIIb or β3 gene inactivation have a Glanzmann thrombasthenia phenotype with bleeding linked to surgery, an increased bleeding time and an absence of platelet aggregation and thrombus formation under flow conditions.89,90 In addition, an immunodeficiency syndrome called leukocyte adhesion deficiency (LAD)-III induces a Glanzmann thrombasthenia-like bleeding disorder due to a mutation of the kindlin-3 gene in hematopoietic cells: platelet surface expression of αIIbβ3 integrin is normal but the mutation causes a defect in β3 integrin activation, inducing a decrease in fibrinogen binding and platelet aggregation.91 Contrary to Glanzmann patients, platelets from LAD-III patients have an adhesion defect on immobilized collagen in perfusion assays since α2β1 integrin is unable to bind collagen due to the kind-lin-3 mutation.92 In sharp contrast, integrin αvβ3 does not seem to play a major part in hemostasis. This is supported by the modest role of this integrin in platelet functions, but also because no mutation of αvβ3 has been reported to be responsible for a hemorrhagic condition in man.
The importance of platelet integrins in arterial thrombosis
In humans, the presence of an allele inducing enhanced expression of α2β1 has been described to be associated with an increased risk of myocardial infarction, diabetic retinopathy and stroke,93,94 potentially linked to increased platelet adhesion. Consistent with these clinical results, platelet β1 integrins were reported to play a role in experimental thrombosis, as PF4Cre-β1-/- mice showed reduced thrombosis in in vivo models based on a mechanical- or laser-induced lesion;36 however, such protection was not observed in an independent study relying on mechanical injury of the carotid artery, challenging the view of a key role of platelet β1 integrins in arterial thrombosis.95 To date, no study has compared the relative importance of individual integrins in experimental thrombosis. There is some controversy over the importance of α2β1 in experimental thrombosis because while some groups identified a role for this integrin in models relying on injuries induced by rose Bengal and ferric chloride,96,97 others did not observe any protection after a mechanical injury.95 In agreement with the study by Grüner and colleagues, our group also did not identify any obvious role after either injury of the carotid artery induced by ferric chloride (Online Supplementary Figure S2A, B) or deep laser injury of mes-enteric arteries in α2-deficient mice (Online Supplementary Figure S2C, D). We recently reported that α5β1 does not seem to play a major role in arterial thrombosis as PF4Cre-α5-/- mice did not display reduced thrombosis in three different in vivo models (ferric chloride injury of the carotid artery, mechanical lesion of the abdominal aorta and laser lesion of mesenteric arterioles).35 Concerning α6β1, our group observed that PF4Cre-α6-/- mice are protected against thrombosis in in vivo models using a mechanical or laser lesion.36 This protection reached the same level as in the PF4Cre-β1-/- mice mentioned above,36 suggesting that the platelet β1 integrin with greatest importance in arterial thrombosis is α6β1. With regard to β3 integrins, the role of αvβ3 in thrombosis is poorly defined but would seem to be minor, since PF4Cre-αv-/- mice display a normal response in experimental models of thrombosis (Online Supplementary Figure S2E, F). In contrast, the other platelet β3 integrin, αIIbβ3, is undoubtedly the most crucial platelet integrin in arterial thrombosis, because of its ability to ensure platelet aggregation and thrombus growth and stability.89,90 Its importance in thrombosis is attested by the existence of a class of clinical antiplatelet agents targeting this integrin.
Integrins as antithrombotic targets
Integrin αIIbβ3 is a well-established antithrombotic target and three antiplatelet agents currently used in clinical practice target this receptor. Abciximab is a Fab fragment from a chimeric monoclonal antibody that interacts with the ligand-binding site of the β3 chain, but also with the KQAGDV sequence of the αIIb chain, which impairs ligand interaction with αIIbβ3 through a conformational effect.98 Eptifibatide is a cyclic heptapeptide containing the KGD sequence99 and tirofiban is a synthetic non-peptidic molecule derived from tyrosine, both obtained from snake venom disintegrins. They both interact with the binding pocket between the αIIb and β3 chains, blocking fibrinogen and vWF binding.98 All three antiplatelet agents are administered intravenously in emergency situations such as myocardial infarction and percutaneous coronary interventions because they are highly antithrombotic. To expand the use of anti-αIIbβ3 molecules towards the prevention of cardiovascular disease, oral anti-αIIbβ3 preparations have been developed. However, unacceptable side effects were observed, notably increased hemorrhage and mortality, ending the clinical trials.100 This increased mortality was described to be linked to a paradoxical platelet activation by oral αIIbβ3 antagonists which bind to the receptor and promotes its activation.101 Current anti-αIIbβ3 agents target this integrin in its resting and activated states, resulting in an enhanced risk of bleeding. To reduce this risk, it has been proposed that drugs targeting only activated αIIbβ3 could be used. Such agents would have the advantage of not targeting resting αIIbβ3, thereby avoiding their pre-activation, and would only block the activated pro-thrombotic form of integrins. The potential of such agents has been nicely described by the group of Karlheinz Peter, who showed that a single-chain anti-activated αIIbβ3 antibody, SCE5, impaired experimental thrombosis with a minimal effect on hemostasis.102 This type of agents currently includes variable fragments of blocking anti-activated αIIbβ3 antibodies,103 small molecules interfering with the metal ion-dependent adhesion site of β3104 and agents targeting the outside-in signaling of the integrin rather than the integrin itself.105 There are currently no antithrombotic agents targeting any of the other four platelet integrins. Since αvβ3 and α5β1 do not play major roles in experimental arterial thrombosis35 (Online Supplementary Figure S2E, F), they are unlikely to be suitable targets for antithrombotic therapy. With regard to integrin α2β1, a reduction in thrombus formation was reported,96,97 indicating that this receptor could represent a potential antithrombotic target. However, α2β1 is not platelet-specific and blocking this receptor might have side effects. In addition, the observation of embolization in mice deficient in α2β1 suggests that targeting this integrin could be harmful.97 Finally, platelet α6β1 plays an important part in experimental thrombosis but not in hemostasis as PF4Cre-α6-/- mice have a normal bleeding time.36 Hence α6β1 might represent a new and safer antithrombotic target as compared to αIIbβ3. Nevertheless, since α6β1 is not only expressed on platelets but also on endothelial cells106 and is implicated in epithelial anchoring,107 targeting this receptor could have side effects.
The importance of platelet integrins beyond hemostasis
Tumor metastasis
A role of platelets in tumor metastasis has been suggested since thrombocytosis is often observed in cancer patients.108 Some cancer cells, such as ovarian cancer cells, can express interleukin-6, thereby inducing the synthesis of a regulator of platelet production, thrombopoietin, directly by the tumor cells or in the liver. Moreover, in rodent models of experimental metastasis, thrombocytopenia reduced the ability of tumor cells to colonize the lungs, which was restored by platelet transfusion.11 The involvement of platelet β1 integrins has been proposed since mice deficient in all platelet β1 integrins (PF4Cre-β1-/-) developed less lung metastasis than control animals in models of orthotopic metastasis or intravenous tumor cell injection.109 This effect seemed to be mostly due to platelet α6β1 as PF4Cre-α6-/- and PF4Cre-β1-/- mice presented very similar results in both experimental models. A possible mechanism could depend on an interaction between platelet α6β1 and ADAM-9 on tumor cells,109 which would form a platelet shield around the cells, preventing the deleterious effects of high shear stress and helping the cells to escape immune surveillance.110 Platelet α2β1 integrin has been described to play a role in epithelial-mesenchymal transition, a process stimulating the invasive properties of tumor cells.111 Interaction of α2β1 with MCF-7 breast cancer cells caused the cells to secrete transforming growth factor-β1,112 a cytokine known to promote epithelial-mesenchymal transition. As far as concerns platelet β3 integrins, the use of αIIbβ3 inhibitors and transplantation of bone marrow from β3-deficient mice into irradiated wild-type mice have been reported to decrease experimental metastasis.113,114 αIIbβ3 could be implicated in the formation of a shield around tumor cells through platelet αIIbβ3-tumor cell αvβ3 interaction via a ligand (fibrinogen, vWF or throm-bospondin) acting as a bridge between the two integrins,115 as in the α6β1-ADAM-9 interaction. However, the involvement of αIIbβ3 is controversial since in a mouse model of lung colonization, 10 days after inoculation, αIIb-deficient mice developed more metastasis than control animals.116
Sepsis
Platelets have also been described to play a role in sepsis, a life-threatening organ dysfunction caused by infection-induced dysregulation of the inflammatory response leading to a pro-inflammatory state. Thrombocytopenia is present in 20 to 60% of septic patients117 and negatively influences the prognosis.12 In line with the observation of a potential benefit of platelets in sepsis, it has been demonstrated that thrombocytopenia promotes the growth and dissemination of bacteria and increases systemic inflammation, tissue damage and mortality during experimental sepsis in mice.118 However, platelet αIIbβ3 would appear to have a deleterious effect under septic conditions, since anti-αIIbβ3 agents decrease the activation of coagulation, endothelial dysfunction and tissue injury characteristic of sepsis. Thus, eptifibatide has been shown to reduce mortality and improve clinical indicators in experimental mouse models of sepsis.119 Another anti-αIIbβ3 molecule, AZ-1, decreased endothelial cell injury and mortality in a model of endotoxin shock in rabbits.120
Table 2.
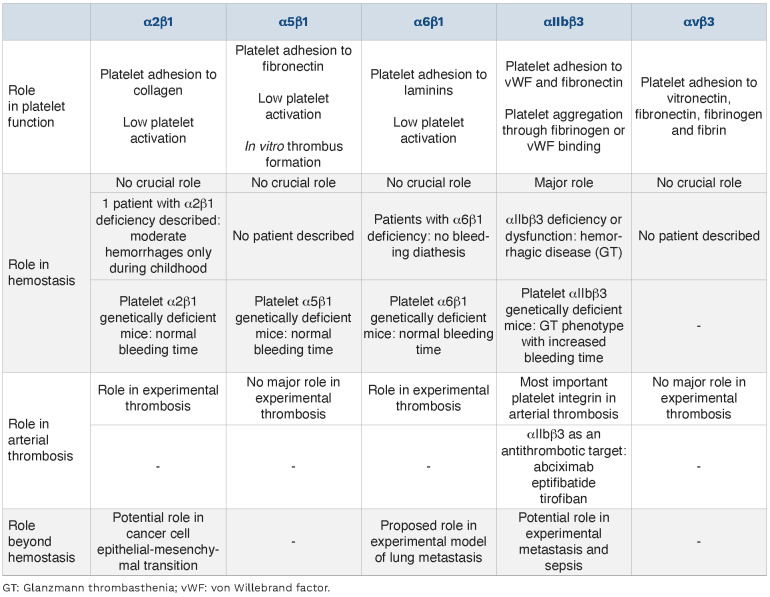
Platelet integrins in platelet function, hemostasis, arterial thrombosis and beyond.
Finally, abciximab reduced sepsis-induced organ damage in a baboon model.121 No role in sepsis has been identified to date for platelet β1 integrins. In brief, as yet there are not enough data to draw conclusions on a deleterious effect of platelet integrins in sepsis and further studies need to be carried out, including in genetically deficient mice.
Alongside their involvement in tumor metastasis and sepsis, platelets have been suggested to be implicated in rheumatoid arthritis and systemic lupus erythemato-sus,13,122 but no role of platelet integrins has yet been described in these diseases.
Conclusions
The main role of platelet integrins is to form a hemo-static plug by participating in: (i) platelet adhesion at the vascular wall; (ii) platelet activation, even though the activation level stays low as compared to that induced in response to soluble agonists; and (iii) platelet aggregation, ensured by αIIbβ3, with a secondary role for α5β1. Platelet αIIbβ3 plays a major role in hemostasis as evidenced by Glanzmann thrombasthenia, with platelet β1 integrins also being implicated in this process. Concerning arterial thrombosis, as for hemostasis, the main platelet integrin implicated is αIIbβ3, with platelet β1 integrins also playing a role, probably mostly through α6β1. Besides these classical roles, platelet integrins have also been described to be implicated in non-hemo-static processes such as tumor metastasis and sepsis. The role of platelet integrins in hemostasis, arterial thrombosis and beyond are summarized in Table 2. Further studies are required to better comprehend the implication of platelet integrins and potentially improve the treatment of these diseases.
Supplementary Material
Acknowledgments
The authors would like to thank Monique Freund and Catherine Ziessel for their help with thrombosis models.
Funding Statement
Funding: This work was supported by INSERM, EFS and ARMESA (Association de Recherche et Développement en Médecine et Santé Publique).
References
- 1.Pease DC. An electron microscopic study of red bone marrow. Blood. 1956;11(6):501-526. [PubMed] [Google Scholar]
- 2.Jackson SP. Arterial thrombosis--insidious, unpredictable and deadly. Nat Med. 2011;17(11):1423-1436. [DOI] [PubMed] [Google Scholar]
- 3.Kitchens CS, Weiss L. Ultrastructural changes of endothelium associated with thrombocytopenia. Blood. 1975;46(4):567-578. [PubMed] [Google Scholar]
- 4.Gupta S, Konradt C, Corken A, et al. Hemostasis vs. homeostasis: platelets are essential for preserving vascular barrier function in the absence of injury or inflammation. Proc Natl Acad Sci U S A. 2020;117(39):24316-24325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goerge T, Ho-Tin-Noe B, Carbo C, et al. Inflammation induces hemorrhage in thrombocytopenia. Blood. 2008;111(10):4958-4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nieswandt B, Watson SP. Platelet-collagen interaction: is GPVI the central receptor? Blood. 2003;102(2):449-461. [DOI] [PubMed] [Google Scholar]
- 7.Nieswandt B, Brakebusch C, Bergmeier W, et al. Glycoprotein VI but not alpha2beta1 integrin is essential for platelet interaction with collagen. EMBO J. 2001;20(9):2120-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Versteeg HH, Heemskerk JWM, Levi M, Reitsma PH. New fundamentals in hemostasis. Physiol Rev. 2013;93(1):327-358. [DOI] [PubMed] [Google Scholar]
- 9.Haining EJ, Lowe KL, Wichaiyo S, et al. Lymphatic blood filling in CLEC-2-deficient mouse models. Platelets. 2021;32(3):352-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yeaman MR. Platelets: at the nexus of antimicrobial defence. Nat Rev Microbiol. 2014;12(6):426-437. [DOI] [PubMed] [Google Scholar]
- 11.Gasic GJ, Gasic TB, Stewart CC. Antimetastatic effects associated with platelet reduction. Proc Natl Acad Sci U S A. 1968;61(1):46-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Claushuis TAM, van Vught LA, Scicluna BP, et al. Thrombocytopenia is associated with a dysregulated host response in critically ill sepsis patients. Blood. 2016;127(24):3062-3072. [DOI] [PubMed] [Google Scholar]
- 13.Boilard E, Nigrovic PA, Larabee K, et al. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science. 2010;327(5965):580-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joseph JE, Harrison P, Mackie IJ, Isenberg DA, Machin SJ. Increased circulating platelet-leucocyte complexes and platelet activation in patients with antiphospholipid syndrome, systemic lupus erythematosus and rheumatoid arthritis. Br J Haematol. 2001;115(2):451-459. [DOI] [PubMed] [Google Scholar]
- 15.Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res. 2010;339(1):269-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang K, Chen J. The regulation of integrin function by divalent cations. Cell Adhes Migr. 2012;6(1):20-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu P, Luo B-H. Integrin αIIbβ3 transmembrane domain separation mediates bi-directional signaling across the plasma membrane. PloS One. 2015;10(1):e0116208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takagi J, Petre BM, Walz T, Springer TA. Global conformational rearrangements in integrin extracellular domains in outside-in and inside-out signaling. Cell. 2002;110(5):599-511. [DOI] [PubMed] [Google Scholar]
- 19.Carman CV, Springer TA. Integrin avidity regulation: are changes in affinity and conformation underemphasized? Curr Opin Cell Biol. 2003;15(5):547-556. [DOI] [PubMed] [Google Scholar]
- 20.Piotrowicz RS, Orchekowski RP, Nugent DJ, Yamada KY, Kunicki TJ. Glycoprotein Ic-IIa functions as an activation-independent fibronectin receptor on human platelets. J Cell Biol. 1988;106(4):1359-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sonnenberg A, Modderman PW, Hogervorst F. Laminin receptor on platelets is the integrin VLA-6. Nature. 1988;336(6198):487-489. [DOI] [PubMed] [Google Scholar]
- 22.Staatz WD, Rajpara SM, Wayner EA, Carter WG, Santoro SA. The membrane glycoprotein Ia-IIa (VLA-2) complex mediates the Mg++-dependent adhesion of platelets to collagen. J Cell Biol. 1989;108(5):1917-1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang X, Chitalia SV, Matsuura S, Ravid K. Integrins and their role in megakaryocyte development and function. Exp Hematol. 2022;106:31-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schick PK, Wojenski CM, He X, Walker J, Marcinkiewicz C, Niewiarowski S. Integrins involved in the adhesion of megakaryocytes to fibronectin and fibrinogen. Blood. 1998;92(8):2650-2656. [PubMed] [Google Scholar]
- 25.Pan R, Wang J, Nardi MA, Li Z. The inhibition effect of anti-GPIIIa49-66 antibody on megakaryocyte differentiation. Thromb Haemost. 2011;106(3):484-490. [DOI] [PubMed] [Google Scholar]
- 26.Matsuura S, Thompson CR, Ng SK, et al. Adhesion to fibronectin via α5β1 integrin supports expansion of the megakaryocyte lineage in primary myelofibrosis. Blood. 2020;135(25):2286-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leven RM. Differential regulation of integrin-mediated proplatelet formation and megakaryocyte spreading. J Cell Physiol. 1995;163(3):597-607 [DOI] [PubMed] [Google Scholar]
- 28.Avraham H, Cowley S, Chi SY, Jiang S, Groopman JE. Characterization of adhesive interactions between human endothelial cells and megakaryocytes. J Clin Invest. 1993;91(6):2378-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmitz B, Thiele J, Otto F, et al. Evidence for integrin receptor involvement in megakaryocyte-fibroblast interaction: a possible pathomechanism for the evolution of myelofibrosis. J Cell Physiol. 1998;176(3):445-455. [DOI] [PubMed] [Google Scholar]
- 30.Mossuz P, Schweitzer A, Molla A, Berthier R. Expression and function of receptors for extracellular matrix molecules in the differentiation of human megakaryocytes in vitro. Br J Haematol. 1997;98(4):819-827 [DOI] [PubMed] [Google Scholar]
- 31.Matsunaga T, Fukai F, Kameda T, et al. Potentiated activation of VLA-4 and VLA-5 accelerates proplatelet-like formation. Ann Hematol. 2012;91(10):1633-1643. [DOI] [PubMed] [Google Scholar]
- 32.Larson MK, Watson SP. Regulation of proplatelet formation and platelet release by integrin alpha IIb beta3. Blood. 2006;108(5):1509-1514. [DOI] [PubMed] [Google Scholar]
- 33.Mazharian A, Thomas SG, Dhanjal TS, Buckley CD, Watson SP. Critical role of Src-Syk-PLCy2 signaling in megakaryocyte migration and thrombopoiesis. Blood. 2010;116(5):793-800. [DOI] [PubMed] [Google Scholar]
- 34.Habart D, Cheli Y, Nugent DJ, Ruggeri ZM, Kunicki TJ. Conditional knockout of integrin α2β1 in murine megakaryocytes leads to reduced mean platelet volume. PloS One. 2013;8(1):e55094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janus-Bell E, Yakusheva A, Scandola C, et al. Characterization of the role of integrin α5β1 in platelet function, hemostasis, and experimental thrombosis. Thromb Haemost. 2022;122(5):767-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schaff M, Tang C, Maurer E, et al. Integrin α6β1 is the main receptor for vascular laminins and plays a role in platelet adhesion, activation, and arterial thrombosis. Circulation. 2013;128(5):541-552. [DOI] [PubMed] [Google Scholar]
- 37.Kashiwagi H, Kunishima S, Kiyomizu K, et al. Demonstration of novel gain-of-function mutations of αIIbβ3: association with macrothrombocytopenia and Glanzmann thrombasthenia-like phenotype. Mol Genet Genomic Med. 2013;1(2):77-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Savage B, Shattil SJ, Ruggeri ZM. Modulation of platelet function through adhesion receptors. A dual role for glycoprotein IIb-IIIa (integrin alpha IIb beta 3) mediated by fibrinogen and glycoprotein Ib-von Willebrand factor. J Biol Chem. 1992;267(16):11300-11306. [PubMed] [Google Scholar]
- 39.Santoro SA, Walsh JJ, Staatz WD, Baranski KJ. Distinct determinants on collagen support alpha 2 beta 1 integrin-mediated platelet adhesion and platelet activation. Cell Regul. 1991;2(11):905-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Savage B, Saldívar E, Ruggeri ZM. Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand factor. Cell. 1996;84(2):289-297. [DOI] [PubMed] [Google Scholar]
- 41.Siedlecki CA, Lestini BJ, Kottke-Marchant KK, Eppell SJ, Wilson DL, Marchant RE. Shear-dependent changes in the three-dimensional structure of human von Willebrand factor. Blood. 1996;88(8):2939-2950. [PubMed] [Google Scholar]
- 42.Williams RL. Mammalian phosphoinositide-specific phospholipase C. Biochim Biophys Acta. 1999;1441(2-3):255-267. [DOI] [PubMed] [Google Scholar]
- 43.Cifuni SM, Wagner DD, Bergmeier W. CalDAG-GEFI and protein kinase C represent alternative pathways leading to activation of integrin alphaIIbbeta3 in platelets. Blood. 2008;112(5):1696-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lagarrigue F, Paul DS, Gingras AR, et al. Talin-1 is the principal platelet Rap1 effector of integrin activation. Blood. 2020;136(10):1180-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nieswandt B, Moser M, Pleines I, et al. Loss of talin1 in platelets abrogates integrin activation, platelet aggregation, and thrombus formation in vitro and in vivo. J Exp Med. 2007;204(13):3113-3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petrich BG, Fogelstrand P, Partridge AW, et al. The antithrombotic potential of selective blockade of talin-dependent integrin alpha IIb beta 3 (platelet GPIIb-IIIa) activation. J Clin Invest. 2007;117(8):2250-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moser M, Nieswandt B, Ussar S, Pozgajova M, Fässler R. Kindlin-3 is essential for integrin activation and platelet aggregation. Nat Med. 2008;14(3):325-330. [DOI] [PubMed] [Google Scholar]
- 48.Honda S, Shirotani-Ikejima H, Tadokoro S, et al. Integrin-linked kinase associated with integrin activation. Blood. 2009;113(21):5304-5313. [DOI] [PubMed] [Google Scholar]
- 49.Kasirer-Friede A, Kang J, Kahner B, Ye F, Ginsberg MH, Shattil SJ. ADAP interactions with talin and kindlin promote platelet integrin αIIbβ3 activation and stable fibrinogen binding. Blood. 2014;123(20):3156-3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gao J, Huang M, Lai J, et al. Kindlin supports platelet integrin αIIbβ3 activation by interacting with paxillin. J Cell Sci. 2017;130(21):3764-3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Battram AM, Durrant TN, Agbani EO, et al. The phosphatidylinositol 3,4,5-trisphosphate (PI(3,4,5)P3) binder Rasa3 regulates phosphoinositide 3-kinase (PI3K)-dependent integrin αIIbβ3 outside-in signaling. J Biol Chem. 2017;292(5):1691-1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hensch NR, Karim ZA, Druey KM, Tansey MG, Khasawneh FT. RGS10 negatively regulates platelet activation and thrombogenesis. PloS One. 2016;11(11):e0165984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hernandez KR, Karim ZA, Qasim H, Druey KM, Alshbool FZ, Khasawneh FT. Regulator of G-protein signaling 16 is a negative modulator of platelet function and thrombosis. J Am Heart Assoc. 2019;8(5):e011273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tadokoro S, Nakazawa T, Kamae T, et al. A potential role for α-actinin in inside-out αIIbβ3 signaling. Blood. 2011;117(1):250-258. [DOI] [PubMed] [Google Scholar]
- 55.Yuan W, Leisner TM, McFadden AW, et al. CIB1 is an endogenous inhibitor of agonist-induced integrin alphaIIbbeta3 activation. J Cell Biol. 2006;172(2):169-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Petrich BG, Marchese P, Ruggeri ZM, et al. Talin is required for integrin-mediated platelet function in hemostasis and thrombosis. J Exp Med. 2007;204(13):3103-3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jung SM, Moroi M. Platelet collagen receptor integrin alpha2beta1 activation involves differential participation of ADP-receptor subtypes P2Y1 and P2Y12 but not intracellular calcium change. Eur J Biochem. 2001;268(12):3513-3522. [DOI] [PubMed] [Google Scholar]
- 58.Pula G, Poole AW. Critical roles for the actin cytoskeleton and cdc42 in regulating platelet integrin alpha2beta1. Platelets. 2008;19(3):199-210. [DOI] [PubMed] [Google Scholar]
- 59.Harburger DS, Bouaouina M, Calderwood DA. Kindlin-1 and -2 directly bind the C-terminal region of beta integrin cytoplasmic tails and exert integrin-specific activation effects. J Biol Chem. 2009;284(17):11485-11497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Obergfell A, Eto K, Mocsai A, et al. Coordinate interactions of Csk, Src, and Syk kinases with αIIbβ3 initiate integrin signaling to the cytoskeleton. J Cell Biol. 2002;157(2):265-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arias-Salgado EG, Haj F, Dubois C, et al. PTP-1B is an essential positive regulator of platelet integrin signaling. J Cell Biol. 2005;170(5):837-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Inoue O, Suzuki-Inoue K, Dean WL, Frampton J, Watson SP. Integrin alpha2beta1 mediates outside-in regulation of platelet spreading on collagen through activation of Src kinases and PLCgamma2. J Cell Biol. 2003;160(5):769-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Obergfell A, Judd BA, del Pozo MA, Schwartz MA, Koretzky GA, Shattil SJ. The molecular adapter SLP-76 relays signals from platelet integrin alphaIIbbeta3 to the actin cytoskeleton. J Biol Chem. 2001;276(8):5916-5923. [DOI] [PubMed] [Google Scholar]
- 64.Wonerow P, Pearce AC, Vaux DJ, Watson SP. A critical role for phospholipase Cgamma2 in alphaIIbbeta3-mediated platelet spreading. J Biol Chem. 2003;278(39):37520-37529. [DOI] [PubMed] [Google Scholar]
- 65.Nesbitt WS, Kulkarni S, Giuliano S, et al. Distinct glycoprotein Ib/V/IX and integrin alpha IIbbeta 3-dependent calcium signals cooperatively regulate platelet adhesion under flow. J Biol Chem. 2002;277(4):2965-2972. [DOI] [PubMed] [Google Scholar]
- 66.Inoue O, Suzuki-Inoue K, McCarty OJT, et al. Laminin stimulates spreading of platelets through integrin alpha6beta1-dependent activation of GPVI. Blood. 2006;107(4):1405-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Watson SP, Auger JM, McCarty OJT, Pearce AC. GPVI and integrin alphaIIb beta3 signaling in platelets. J Thromb Haemost. 2005;3(8):1752-1762. [DOI] [PubMed] [Google Scholar]
- 68.Cipolla L, Consonni A, Guidetti G, et al. The proline-rich tyrosine kinase Pyk2 regulates platelet integrin αIIbβ3 outside-in signaling. J Thromb Haemost. 2013;11(2):345-356. [DOI] [PubMed] [Google Scholar]
- 69.Hughan SC, Watson SP. Differential regulation of adapter proteins Dok2 and Dok1 in platelets, leading to an association of Dok2 with integrin alphaIIbbeta3. J Thromb Haemost. 2007;5(2):387-394. [DOI] [PubMed] [Google Scholar]
- 70.Law DA, Nannizzi-Alaimo L, Phillips DR. Outside-in integrin signal transduction. Alpha IIb beta 3-(GP IIb IIIa) tyrosine phosphorylation induced by platelet aggregation. J Biol Chem. 1996;271(18):10811-10815. [DOI] [PubMed] [Google Scholar]
- 71.Jenkins AL, Nannizzi-Alaimo L, Silver D, et al. Tyrosine phosphorylation of the beta3 cytoplasmic domain mediates integrin-cytoskeletal interactions. J Biol Chem. 1998;273(22):13878-13885. [DOI] [PubMed] [Google Scholar]
- 72.Gong H, Shen B, Flevaris P, et al. G protein subunit Galpha13 binds to integrin alphaIIbbeta3 and mediates integrin “outside-in” signaling. Science. 2010;327(5963):340-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boylan B, Gao C, Rathore V, Gill JC, Newman DK, Newman PJ. Identification of FcgammaRIIa as the ITAM-bearing receptor mediating alphaIIbbeta3 outside-in integrin signaling in human platelets. Blood. 2008;112(7):2780-2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhi H, Rauova L, Hayes V, et al. Cooperative integrin/ITAM signaling in platelets enhances thrombus formation in vitro and in vivo. Blood. 2013;121(10):1858-1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ahmed MU, Receveur N, Janus-Bell E, et al. Respective roles of glycoprotein VI and FcyRIIA in the regulation of αIIbp3-mediated platelet activation to fibrinogen, thrombus buildup, and stability. Res Pract Thromb Haemost. 2021;5(5):e12551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ahmed MU, Kaneva V, Loyau S, et al. Pharmacological blockade of glycoprotein VI promotes thrombus disaggregation in the absence of thrombin. Arterioscler Thromb Vasc Biol. 2020;40(9):2127-2142. [DOI] [PubMed] [Google Scholar]
- 77.Mangin PH, Onselaer M-B, Receveur N, et al. Immobilized fibrinogen activates human platelets through GPVI. Haematologica. 2018;103(9):1568-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Janus-Bell E, Ahmed MU, Receveur N, et al. Differential role of glycoprotein VI in mouse and human thrombus progression and stability. Thromb Haemost. 2021;121(4):543-546. [DOI] [PubMed] [Google Scholar]
- 79.Panteleev MA, Korin N, Reesink KD, et al. Wall shear rates in human and mouse arteries: Standardization of hemodynamics for in vitro blood flow assays: communication from the ISTH SSC Subcommittee on Biorheology. J Thromb Haemost. 2021;19(2):588-595. [DOI] [PubMed] [Google Scholar]
- 80.Maurer E, Schaff M, Receveur N, et al. Fibrillar cellular fibronectin supports efficient platelet aggregation and procoagulant activity. Thromb Haemost. 2015;114(6):1175-1188. [DOI] [PubMed] [Google Scholar]
- 81.De Marco L, Girolami A, Zimmerman TS, Ruggeri ZM. von Willebrand factor interaction with the glycoprotein IIb/IIa complex. Its role in platelet function as demonstrated in patients with congenital afibrinogenemia. J Clin Invest. 1986;77(4):1272-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kulkarni S, Dopheide SM, Yap CL, et al. A revised model of platelet aggregation. J Clin Invest. 2000;105(6):783-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ruggeri ZM, Orje JN, Habermann R, Federici AB, Reininger AJ. Activation-independent platelet adhesion and aggregation under elevated shear stress. Blood. 2006;108(6):1903-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nieuwenhuis HK, Sakariassen KS, Houdijk WP, Nievelstein PF, Sixma JJ. Deficiency of platelet membrane glycoprotein Ia associated with a decreased platelet adhesion to subendothelium: a defect in platelet spreading. Blood. 1986;68(3):692-695. [PubMed] [Google Scholar]
- 85.Holtkötter O, Nieswandt B, Smyth N, et al. Integrin alpha 2-deficient mice develop normally, are fertile, but display partially defective platelet interaction with collagen. J Biol Chem. 2002;277(13):10789-10794. [DOI] [PubMed] [Google Scholar]
- 86.Nieswandt B, Brakebusch C, Bergmeier W, et al. Glycoprotein VI but not alpha2beta1 integrin is essential for platelet interaction with collagen. EMBO J. 2001;20(9):2120-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Petzold T, Ruppert R, Pandey D, et al. β1 integrin-mediated signals are required for platelet granule secretion and hemostasis in mouse. Blood. 2013;122(15):2723-2731. [DOI] [PubMed] [Google Scholar]
- 88.Solh T, Botsford A, Solh M. Glanzmann’s thrombasthenia: pathogenesis, diagnosis, and current and emerging treatment options. J Blood Med. 2015;6:219-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hodivala-Dilke KM, McHugh KP, Tsakiris DA, et al. Beta3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J Clin Invest. 1999;103(2):229-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tronik-Le Roux D, Roullot V, Poujol C, Kortulewski T, Nurden P, Marguerie G. Thrombasthenic mice generated by replacement of the integrin alpha(IIb) gene: demonstration that transcriptional activation of this megakaryocytic locus precedes lineage commitment. Blood. 2000;96(4):1399-1408. [PubMed] [Google Scholar]
- 91.Wolach B, Gavrieli R, Wolach O, et al. Leucocyte adhesion deficiency-a multicentre national experience. Eur J Clin Invest. 2019;49(2):e13047. [DOI] [PubMed] [Google Scholar]
- 92.van de Vijver E, De Cuyper IM, Gerrits AJ, et al. Defects in Glanzmann thrombasthenia and LAD-III (LAD-1/v) syndrome: the role of integrin β1 and β3 in platelet adhesion to collagen. Blood. 2012;119(2):583-586. [DOI] [PubMed] [Google Scholar]
- 93.Matsubara Y, Murata M, Maruyama T, et al. Association between diabetic retinopathy and genetic variations in alpha2beta1 integrin, a platelet receptor for collagen. Blood. 2000;95(5):1560-1564. [PubMed] [Google Scholar]
- 94.Santoso S, Kunicki TJ, Kroll H, Haberbosch W, Gardemann A. Association of the platelet glycoprotein Ia C807T gene polymorphism with nonfatal myocardial infarction in younger patients. Blood. 1999;93(8):2449-2453. [PubMed] [Google Scholar]
- 95.Grüner S, Prostredna M, Schulte V, et al. Multiple integrin-ligand interactions synergize in shear-resistant platelet adhesion at sites of arterial injury in vivo. Blood. 2003;102(12):4021-4027. [DOI] [PubMed] [Google Scholar]
- 96.He L, Pappan LK, Grenache DG, et al. The contributions of the alpha 2 beta 1 integrin to vascular thrombosis in vivo. Blood. 2003;102(10):3652-3657. [DOI] [PubMed] [Google Scholar]
- 97.Kuijpers MJE, Pozgajova M, Cosemans JMEM, et al. Role of murine integrin alpha2beta1 in thrombus stabilization and embolization: contribution of thromboxane A2. Thromb Haemost. 2007;98(5):1072-1080. [PubMed] [Google Scholar]
- 98.Hashemzadeh M, Furukawa M, Goldsberry S, Movahed MR. Chemical structures and mode of action of intravenous glycoprotein IIb/IIIa receptor blockers: a review. Exp Clin Cardiol. 2008;13(4):192-197. [PMC free article] [PubMed] [Google Scholar]
- 99.Phillips DR, Scarborough RM. Clinical pharmacology of eptifibatide. Am J Cardiol. 1997;80(4A):11B-20B. [DOI] [PubMed] [Google Scholar]
- 100.Chew DP, Bhatt DL, Sapp S, Topol EJ. Increased mortality with oral platelet glycoprotein IIb/IIIa antagonists: a meta-analysis of phase III multicenter randomized trials. Circulation. 2001;103(2):201-206. [DOI] [PubMed] [Google Scholar]
- 101.Bassler N, Loeffler C, Mangin P, et al. A mechanistic model for paradoxical platelet activation by ligand-mimetic alphaIIb beta3 (GPIIb/IIIa) antagonists. Arterioscler Thromb Vasc Biol. 2007;27(3):e9-15. [DOI] [PubMed] [Google Scholar]
- 102.Schwarz M, Meade G, Stoll P, et al. Conformation-specific blockade of the integrin GPIIb/IIIa: a novel antiplatelet strategy that selectively targets activated platelets. Circ Res. 2006;99(1):25-33. [DOI] [PubMed] [Google Scholar]
- 103.Ziegler M, Hohmann JD, Searle AK, et al. A single-chain antibody-CD39 fusion protein targeting activated platelets protects from cardiac ischaemia/reperfusion injury. Eur Heart J. 2018;39(2):111-116. [DOI] [PubMed] [Google Scholar]
- 104.Li J, Vootukuri S, Shang Y, et al. RUC-4: a novel αIIbβ3 antagonist for prehospital therapy of myocardial infarction. Arterioscler Thromb Vasc Biol. 2014;34(10):2321-2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Huang J, Shi X, Xi W, Liu P, Long Z, Xi X. Evaluation of targeting c-Src by the RGT-containing peptide as a novel antithrombotic strategy. J Hematol Oncol. 2015;8:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Larrieu-Lahargue F, Welm AL, Thomas KR, Li DY. Netrin-4 activates endothelial integrin α6β1. Circ Res. 2011;109(7):770-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kadoya Y, Kadoya K, Durbeej M, Holmvall K, Sorokin L, Ekblom P. Antibodies against domain E3 of laminin-1 and integrin alpha 6 subunit perturb branching epithelial morphogenesis of submandibular gland, but by different modes. J Cell Biol. 1995;129(2):521-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hufnagel DH, Cozzi GD, Crispens MA, Beeghly-Fadiel A. Platelets, thrombocytosis, and ovarian cancer prognosis: surveying the landscape of the literature. Int J Mol Sci. 2020;21(21):8169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mammadova-Bach E, Zigrino P, Brucker C, et al. Platelet integrin α6β1 controls lung metastasis through direct binding to cancer cell-derived ADAM9. JCI Insight. 2016;1(14):e88245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nieswandt B, Hafner M, Echtenacher B, Männel DN. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res. 1999;59(6):1295-1300. [PubMed] [Google Scholar]
- 111.Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20(5):576-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zuo X-X, Yang Y, Zhang Y, Zhang Z-G, Wang X-F, Shi Y-G. Platelets promote breast cancer cell MCF-7 metastasis by direct interaction: surface integrin α2β1-contacting-mediated activation of Wnt-β-catenin pathway. Cell Commun Signal. 2019;17(1):142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Amirkhosravi A, Mousa SA, Amaya M, et al. Inhibition of tumor cell-induced platelet aggregation and lung metastasis by the oral GpIIb/IIIa antagonist XV454. Thromb Haemost. 2003;90(3):549-554. [DOI] [PubMed] [Google Scholar]
- 114.Bakewell SJ, Nestor P, Prasad S, et al. Platelet and osteoclast beta3 integrins are critical for bone metastasis. Proc Natl Acad Sci U S A. 2003;100(24):14205-14210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dardik R, Kaufmann Y, Savion N, Rosenberg N, Shenkman B, Varon D. Platelets mediate tumor cell adhesion to the subendothelium under flow conditions: involvement of platelet GPIIb-IIIa and tumor cell alpha(v) integrins. Int J Cancer. 1997;70(2):201-207. [DOI] [PubMed] [Google Scholar]
- 116.Echtler K, Konrad I, Lorenz M, et al. Platelet GPIIb supports initial pulmonary retention but inhibits subsequent proliferation of melanoma cells during hematogenic metastasis. PloS One. 2017;12(3):e0172788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Greinacher A, Selleng K. Thrombocytopenia in the intensive care unit patient. Hematol Am Soc Hematol Educ Program. 2010;2010:135-143. [DOI] [PubMed] [Google Scholar]
- 118.de Stoppelaar SF, van ’t Veer C, Claushuis TAM, Albersen BJA, Roelofs JJTH, van der Poll T. Thrombocytopenia impairs host defense in Gram-negative pneumonia-derived sepsis in mice. Blood. 2014;124(25):3781-3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sharron M, Hoptay CE, Wiles AA, et al. Platelets induce apoptosis during sepsis in a contact-dependent manner that is inhibited by GPIIb/IIIa blockade. PloS One. 2012;7(7):e41549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pu Q, Wiel E, Corseaux D, et al. Beneficial effect of glycoprotein IIb/IIIa inhibitor (AZ-1) on endothelium in Escherichia coli endotoxin-induced shock. Crit Care Med. 2001;29(6):1181-1188. [DOI] [PubMed] [Google Scholar]
- 121.Taylor FB, Coller BS, Chang AC, et al. 7E3 F(ab’)2, a monoclonal antibody to the platelet GPIIb/IIIa receptor, protects against microangiopathic hemolytic anemia and microvascular thrombotic renal failure in baboons treated with C4b binding protein and a sublethal infusion of Escherichia coli. Blood. 1997;89(11):4078-4084. [PubMed] [Google Scholar]
- 122.Duffau P, Seneschal J, Nicco C, et al. Platelet CD154 potentiates interferon-alpha secretion by plasmacytoid dendritic cells in systemic lupus erythematosus. Sci Transl Med. 2010;2(47):47ra63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.