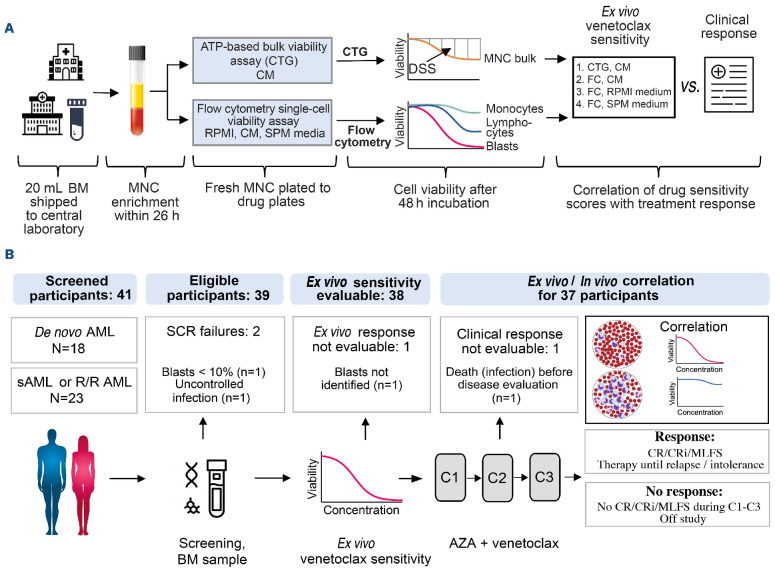
Figure 1.
Sample processing and trial outline. (A) Outline of sample processing and drug sensitivity testing. (B) Outline of study recruitment, including number of participants, reasons for screening failure, and number of participants eligible for ex vivo/in vivo correlation. BM: bone marrow; MNC: mononuclear cells; CTG: CellTiterGlo®; CM: conditioned medium; DSS: drug sensitivity score; FC: flow cytometry; AML: acute myeloid leukemia; sAML: secondary AML; R/R AML: relapsed and/or resistant AML; SCR: screening; C: cycle; AZA: azacitidine; CR: complete remission; CRi: CR with incomplete blood recovery; MLFS: morphological leukemia-free state.