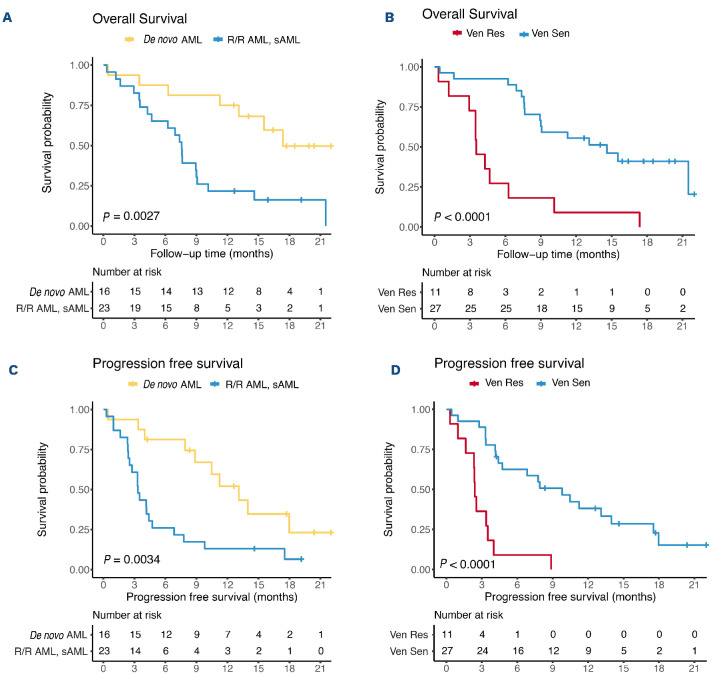
Figure 4.
Overall survival based on disease state and ex vivo sensitivity to venetoclax. (A) The median overall survival for de novo acute myeloid leukemia (AML) versus secondary AML (sAML) or relapsed/refractory AML (R/R AML) was 17.4 months (95% confidence interval [95% CI]: not reached) and 7.6 months (95% CI: 6.5-8.6), respectively. (B) The median overall survival for participants with a drug sensitivity score <10.7 (ex vivo-resistant) versus >10.7 (ex vivo-sensitive) was 3.5 months (95% CI: 2.5-4.6) and 14.6 months (95% CI: 8.8-20.4), respectively. Participants alive at the data cutoff day were censored. The median follow-up time was 18.6 months. (C) The median progression-free survival for de novo AML versus sAML or R/R AML was 13.1 months and 3.3 months, respectively. (D) The median progression-free survival for ex vivo drug-resistant versus ex vivo drug-sensitive patients was 9.8 versus 2.4 months, respectively. Progression-free survival was defined as the number of days from the date of the first dose to the date when the patient was deemed refractory or the earliest evidence of relapse or death. Ven Res: resistant to venetoclax; Ven Sen, sensitive to venetoclax.