Summary
Stress-elevated glucocorticoids cause circadian disturbances and gut-brain axis (GBA) disorders, including irritable bowel syndrome (IBS). We hypothesized that the glucocorticoid receptor (GR/NR3C1) might cause chromatin circadian misalignment in the colon epithelium. We observed significantly decreased core circadian gene Nr1d1 in water avoidance stressed (WAS) BALB/c colon epithelium, like in IBS patients. WAS decreased GR binding at the Nr1d1 promoter E-box (enhancer box), and GR could suppress Nr1d1 via this site. Stress also altered GR binding at the E-box sites along the Ikzf3-Nr1d1 chromatin and remodeled circadian chromatin 3D structures, including Ikzf3-Nr1d1 super-enhancer, Dbp, and Npas2. Intestinal deletion of Nr3c1 specifically abolished these stress-induced transcriptional alternations relevant to IBS phenotypes in BALB/c mice. GR mediated Ikzf3-Nr1d1 chromatin disease related circadian misalignment in stress-induced IBS animal model. This animal model dataset suggests that regulatory SNPs of human IKZF3-NR1D1 transcription through conserved chromatin looping have translational potential based on the GR-mediated circadian-stress crosstalk.
Subject areas: Molecular biology, Neuroscience, Transcriptomics
Graphical abstract
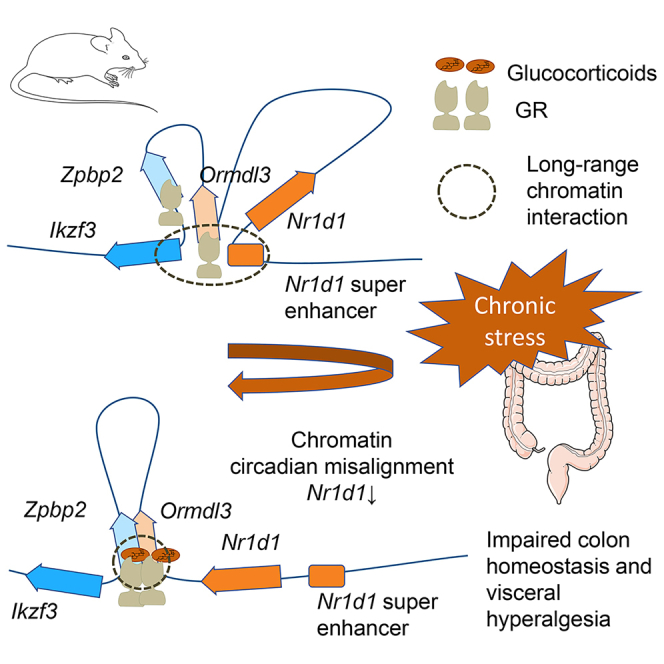
Highlights
-
•
Chronic stress causes chromatin misalignment and impaired colon epithelium homeostasis
-
•
Glucocorticoid receptor programs transcriptional changes via chromatin loops
-
•
Stress-circadian crosstalk via- Ikzf3-Nr1d1 chromatin suggests translational potential
Molecular biology; Neuroscience; Transcriptomics
Introduction
Functional bowel disorders (FBDs), including irritable bowel syndrome (IBS), are now recognized as disorders of gut-brain interaction (DGBI), for differential symptoms may share close pathological mechanisms in the gut-brain axis (GBA). Glucocorticoids (GCs) are responsible for the bidirectional interaction of the GBA.1,2,3 We previously reported that hypothalamic-pituitary-adrenal (HPA) axis-derived endogenous GCs mediate visceral hyperalgesia and barrier dysfunction of colon intestinal epithelial cells (IECs) in a stress-induced IBS animal model through transcriptional regulation of the glucocorticoid receptor (GR/NR3C1).4,5 In addition, previous reports of lower NR1D1 (the core circadian gene encoding REV-ERBα) mRNA levels in IBS patient colonoscopy biopsies led us to propose the existence of a stress-GR-NR1D1 E-box (enhancer box) pathway as a mechanism for IBS as the basis of this pilot study exploring stress-GR modulated 3D genome structure of IECs.2,6,7
The endogenous GCs (cortisol in humans and corticosterone/CORT in rodents) are hormones involved in metabolism, inflammatory responses, cellular proliferation, differentiation, circadian rhythmicity, and stress responses.2 Furthermore, exogenous GCs have been used as anti-inflammatory steroids that synchronize circadian-dependent chromatin 3D structure and IEC homeostasis in vitro.2,8,9 Ileal-derived CORT regulates the oscillatory circadian-transcriptional network consisting of circadian genes Per2, Clock, Arntl (the gene encoding BMAL1), Dbp, and Nr1d1 in maintaining the homeostasis of IECs in a microbiota-dependent manner through GR.1,10 CORT levels are elevated in germ-free mice, with GR playing a pivotal role in the downstream transcriptional regulation in GBA.1,3 Meanwhile, psychological stress elevates blood GCs levels via the HPA axis. GCs bind GR in the cytosol, and GR is translocated to the nucleus, where GR serves as a transcription factor (TF) by binding to glucocorticoid-responsive DNA elements (GREs) in the promoters. This interaction leads to the activation or repression of the transcription of genes involved in the stress responses.2 The circadian hormone melatonin, which can antagonize GR translocation, may alleviate the effect of stress and demonstrate effectiveness in IBS patients.11,12 In addition, GR interacts with enhancer GREs and the cohesin loader nipped-B-like protein (NIPBL) to initiate chromatin loop extrusion as a mechanism for long-distance regulation: GR and NIPBL bind enhancer GREs firstly and load cohesin ring to guide the formation of chromatin loop, then the chromatin slides through the cohesin ring until the formation of stable transcription-regulatory enhancer-promoter interaction via the TF binding DNA elements.13 E-box is a DNA response element targeted by the CLOCK:BMAL1 TF complex in the circadian transcriptional network.14,15 GR also interacts indirectly with E-boxes in the Nr1d1 promoter via the CLOCK:BMAL1 protein complex to repress Nr1d1 during stress.7 Intestinal deletion of Nr1d1 demonstrated that NR1D1 is a crucial regulator of the transcriptome in the mouse colitis (inflammatory bowel disease [IBD]) model; loss of NR1D1 leads to increased colonic inflammation through NLRP3.16 The NLRP3 inflammasome is also an emerging therapeutic target for chronic pain and IBS.17 Moreover, diurnal dynamic interactions between GR and NR1D1 protein modulate their nuclear co-localization by co-binding to the genomic DNA, thereby regulating downstream transcription and NR1D1 coupled GC genomic action.18,19 GR and NR1D1 interact with the NCOR1 (nuclear receptor corepressor 1)-HDAC3 (histone deacetylase 3) complex and mediate GC and circadian-driven chromatin looping, thereby suggesting circadian-stress 4D nucleome (4DN/spatial and temporal changes in the structure of the nucleus) crosstalk.20,21 During the circadian, 5′ Fbxl20 to 3′ Nr1d1 super-enhancer circadian oscillatory chromatin looping was regulated by the BMAL1, NR1D1, and cohesin-CTCF.22 GR may participate in this regulation, and stress may reprogram the Nr1d1 chromatin in 3D.13,14,15
Chromatin 3D structures, including long-distance enhancer-promoter interactions, can help elucidate the pathological mechanism of single nucleotide polymorphisms (SNPs): Most SNPs are located in non-coding areas and so, might serve as enhancers. The PsychENCODE project studying these enhancer-promoter pairs identified NR1D1 as a "master regulator" of targetable transcriptional networks.23 Specifically, regulatory SNPs located in long-range enhancers of differentially expressed GR target genes could predict stress-induced risk-related brain function and psychiatric disorders as well as metabolic effects of GC and GC treatment responses.24,25 Since the workshop “Functional Bowel Disorders: A Roadmap to Guide the Next Generation of Research,” high-quality multi-omics data, including transcriptomic and genome-wide association studies (GWAS) of IBS patients, were generated.26,27,28,29,30,31 However, IBS-related chromatin 3D structure remains elusive.2,23 The latest GWAS of a large IBS population revealed IBS risk loci and SNPs that suggested shared genetic pathways with psychological mood and anxiety disorders; NIPBL was detected in IBS etiology analysis (shown in Supplementary Table 10 of this reference).30 The GR-NIPBL-cohesin target gene TSC22D3 (GILZ/glucocorticoid-induced leucine zipper protein) was the most significant differentially expressed gene (DEG) between IBS-C (constipation) and IBS-D (diarrhea) (false discovery rate (FDR) < 0.005, shown in Table S6 of this reference).13,29 The transcriptional regulation mediated by GR-NIPBL-cohesin is responsible for the response to GC and may also participate in stress-induced IBS.13,32 Capture-C sequencing of human hypothalamic-like neurons and colonoids 3D genomes were performed to explore stress and depression-associated IBD; STARD3 at the 5′-distal region of NR1D1 and the IBS DEG CDH3 (P-cadherin) (IBS-C vs. Healthy Control, FDR <0.05) were identified as putative effector genes.27,28,29,33 This study also revealed a close genetic correlation between depression with IBD and asthma.33 Encoded between STARD3-NR1D1, the hypothesized IBS DEG ORMDL3 shares the IBS SNP rs2872507 with IKZF3, a recently verified IBS DEG with the highest significance (IBS-C vs. IBS-D, right colon p < 0.00001 left colon p < 0.0000001; IBS-D vs. Healthy Control right colon p < 0.01 left colon p < 0.0001).27,28,31 This SNP also regulates IBD DEGs encoded within the conserved 17q12-q21 ERBB2 (HER2)-GRB7-IKZF3-ZPBP2-GSDMB-ORDML3-MED24 chromatin region upstream of NR1D1 and is associated with differential responses to GC therapy against asthma.34,35,36 The asthma-risk variants rs4065275 (ORMDL3 site) and rs12936231 (ZPBP2 site) switch CTCF-binding sites that mediate pathological IKZF3-ORMDL3 to ZPBP2-ORMDL3 chromatin loop changes.37 Thus, the 5′ ZPBP2-ORMDL3 region regulates 3′ NR1D1 circadian transcription in cis from long distances.35 The potential GR-mediated chromatin interactions linking these regulatory SNPs have translational potential in precision medicine against psychological mood and anxiety disorders and IBS.2,23,30 In vitro germ-free colonoids used in the Capture-C sequencing study tend to lose circadian oscillatory transcription without daily GC treatments; it has limitations in studying intestinal epithelium Nr1d1 regulated by circadian and microbiota in vivo.1,9,33,38 To overcome this limitation, we employed a validated water avoidance stressed (WAS) mouse model of IBS to explore 3D genome structure in vivo.2 BALB/c strain used in this study is the preferred strain for circadian-stress crosstalk.39
Results
Stress altered circadian-related transcription responsible for IBS phenotype
We first performed a transcriptomic analysis to identify DEGs responsible for colonic stress responses (Figures 1 and S1). Decreased mRNA levels of Nr1d1 and the NR1D1 downstream clock gene target Dbp were the most significant DEGs that encode TFs (Figures 1A and 1C). The KEGG (Kyoto Encyclopedia of Genes and Genomes) pathway enrichment analysis enriched chemokine signaling, tumor necrosis factor (TNF), and bile acid secretion changes (Figure 1D). Given the emerging evidence of circadian gene Nr1d1 and Dbp in intestinal circadian misalignment impaired intestinal homeostasis, we focused our investigations on these genes.10,14,16,38,40 In our hypothesis-driven analysis, we t tested our interested circadian and IBS-related genes for low-amplitude changes, which were filtered by automated RNA-seq analysis with a 2-fold threshold. In addition to IBS and IBD-related DEGs encoded within the conserved Nr1d1-Ormdl3-Ikzf3-Grb7-Erbb2-Stard3-Fbxl20 chromatin, we observed stress responses in the validated circadian chromatin 3D structure-regulated genes Nr1d2, Npas2, Rorc, and Pard3.8,21,22,31,35,41,42 NR1D1 downstream circadian gene Arntl(Bmal1), Nfil3 elevated.16 IBS therapeutic target homolog Slc9a3 (hydrogen exchanger 3/Nhe3) decreased.43 IBS-related GR target genes Fkbp5 and Fos decreased.3,13,31 IBS DEG homolog Cdh3 increased.29 Elevated Edn1 was identified as an IBS-related DEG with high significance (Figures 1B and 1E).44 We employed BART (binding analysis for regulation of transcription) for potential transcription regulators of WAS-induced DEGs. Within the predicted transcriptional regulators, GR/NR3C1 was the most significant TF and HDAC3 was the most significant chromatin regulator (Figure 1F). Cohesin components SMC1A, SMC3, RAD21, STAG1, and CTCF involved in the GR-mediated promoter-enhancer interaction were also recognized.13 We then predicted the potential transcription network with the most significant 50 transcription regulators from BART analysis and identified the circadian clock as the most similar pathway using Cytoscape; GC can modulate this transcriptional network via GR (Figure 1G). Then we examined downstream circadian disturbances in colon epithelial cells via qPCR at ZT2-ZT26; stress altered the circadian expression of circadian genes and significantly increased Cry1, which is repressed by NR1D1 through NR1D1-mediated enhancer-promoter chromatin looping (Figure S2).21 The transcriptome results were confirmed by Western blot analysis showing decreases in levels of NR1D1 and GR and increased expression of the NR1D1-mediated inflammatory proteins NLRP3, IL1β, and IL6.5,16 In addition, intestinal barrier regulator PARD3 decreased (Figure 2A).45 Furthermore, WAS-induced IBS phenotype, including inflammatory infiltration, barrier dysfunction, and visceral hyperalgesia, were verified. Injections of NR1D1 agonist SR9009 during water avoidance stress significantly prevented these phenotype changes (Figures 2B–2F).16,46 We also evaluated the stress-induced anxiety behavior via the open field test; WAS significantly increased velocity and total distances (Figure S3).47 These findings indicate that WAS BALB/c mice mimic hypothesized stress-GC-GR-NR1D1 IBS pathology and confirm the suitability of this model for further analysis (Figures 1 and 2).2
Figure 1.
Stress-induced colon epithelium DEGs suggest circadian-stress crosstalk
Control (CT) and water avoidance stressed (WAS) BALB/c mouse colon epithelium cells were isolated for RNA-seq analysis.
(A) Principal component analysis.
(B) Volcano plot of DEGs.
(C) Heatmap of transcription factor DEGs.
(D) KEGG pathway enrichment of WAS-induced DEGs.
(E) Heatmap of circadian, GR target, and IBS-related DEGs. Statistical significance was determined using an unpaired t test with Welch’s correction (∗, p < 0.05; ∗∗, p < 0.01; ∗∗∗, p < 0.001; ∗∗∗∗, p < 0.0001; N = 3).
(F) BART (binding analysis for regulation of transcription) analysis of WAS-induced DEGs for potential transcription regulators.
(G)The top 50 transcription regulators were analyzed with Cytoscape, and the circadian clock was identified as the potential stress-modulated transcription network with the highest similarity.
Figure 2.
The WAS-induced transcriptome changes correlated with IBS phenotypes
(A) Western blot analysis of control (CT) and water avoidance stressed (WAS) mouse colon IECs.
(B) Typical hematoxylin-eosin (HE) staining of CT and WAS colon epithelium with/without NR1D1 agonist SR9009 intervention. Inflammatory infiltration was present in WAS mice, and SR9009 significantly prevented morphology changes.
(C) Pathology scores increased in WAS mice, and SR9009 prevented this effect.
(D) Stress increased FD4 (fluorescein isothiocyanate-dextran 4 kDa) permeability, and this effect was antagonized by SR9009 intervention.
(E) Stress reduced the thresholds of pain responses. Data are expressed as means ± standard error, and statistical significance between groups was determined using an unpaired t test with Welch’s correction (∗, p < 0.05; ∗∗, p < 0.01; ∗∗∗, p < 0.001; N = 3, 4, 5).
(F) Visceral hyperalgesia was evaluated with AWR (abdominal withdrawal reflex) scores in response to CRD (colorectal distension). AWR data were analyzed with two-way ANOVA analysis (with Tukey’s multiple comparison test); the significance between WAS/CT and WAS+SR9009/WAS is illustrated (∗, p < 0.05; ∗∗, p < 0.01; N = 4, 5).
GR repressed Nr1d1 via promoter transcription start site E-box
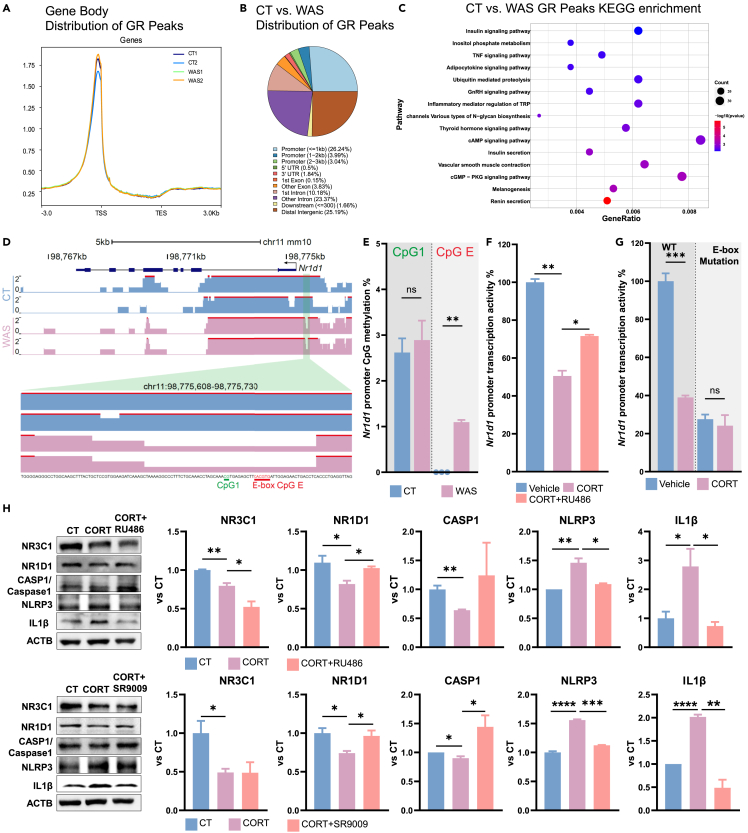
Given that GR is potentially the most significant transcription regulator of WAS-induced transcriptomic responses, we used the highly sensitive CUT&Tag sequencing method to profile genomic GR binding of colonic IECs (Figure 1F). The GR binding peaked at transcription start sites (TSSs) (Figure 3A). WAS caused 33% GR binding changes in promoters, 34% in introns, and 25% in the distal intergenic region (Figure 3B). The changes in the TNF signaling pathway and melanogenesis in KEGG analysis were consistent with transcriptome (Figures 1D and 3C). Specifically, WAS induced a gap in GR binding around the Nr1d1 promoter E-box (Figure 3D). This TSS E-box CpG was 1% methylated in stressed mice, which may have caused the observed gap by blocking the TF binding site (Figure 3E). Within mouse colon crypts derived young adult mouse colon (YAMC) cells, we verified the stress-GC-GR-Nr1d1 promoter E-box pathway responsible for Nr1d1 repression via luciferase assays (Figures 3F and 3G).2,7 In addition, we verified GC’s repression of NR1D1 protein in vitro: NR1D1 was downregulated by CORT, and GR antagonist RU486 prevented this effect. Furthermore, NR1D1 decrease-related elevation in NLRP3 and IL1β was reproduced by CORT treatment and could be prevented by NR1D1 agonist SR9009. These data support a stress-GC-GR-NR1D1-NLRP3 inflammasome pathway in stress-impaired colon epithelium homeostasis (Figure 3H).2,7,16,17
Figure 3.
Stress-induced GR binding change at Nr1d1 TSS E-box
Control (CT) and water avoidance stressed (WAS) mouse colon epithelium cells were isolated for CUT&Tag analysis; hypothesis-free analysis of GR cistrome.
(A) Gene body.
(B) Differential peak annotation.
(C) KEGG pathway enrichment of differential peaks.
(D) Each lane represents IECs pooled from four mice, stress reduced GR binding at the E-box (CACGTG) upstream of the Nr1d1 TSS.
(E) Pyrosequencing analysis of the genomic DNA CpGs within the gap in GR binding around the E-box.
(F and G) Nr1d1 promoter activity in differentiated mouse colon epithelium YAMC cells. 1 μM CORT treatment repressed Nr1d1 transcription, and the GR antagonist RU486 ameliorated this effect. Nr1d1 promoter TSS E-box mutation reduced Nr1d1 transcription.
(H) Western blot analysis of YAMC cells treated with CORT, RU486, and NR1D1 agonist SR9009. Data are expressed as means ± standard error. Statistical significance was determined using an unpaired t test with Welch’s correction (∗, p < 0.05; ∗∗, p < 0.01; ∗∗∗, p < 0.01; ∗∗∗∗, p < 0.0001; N = 3).
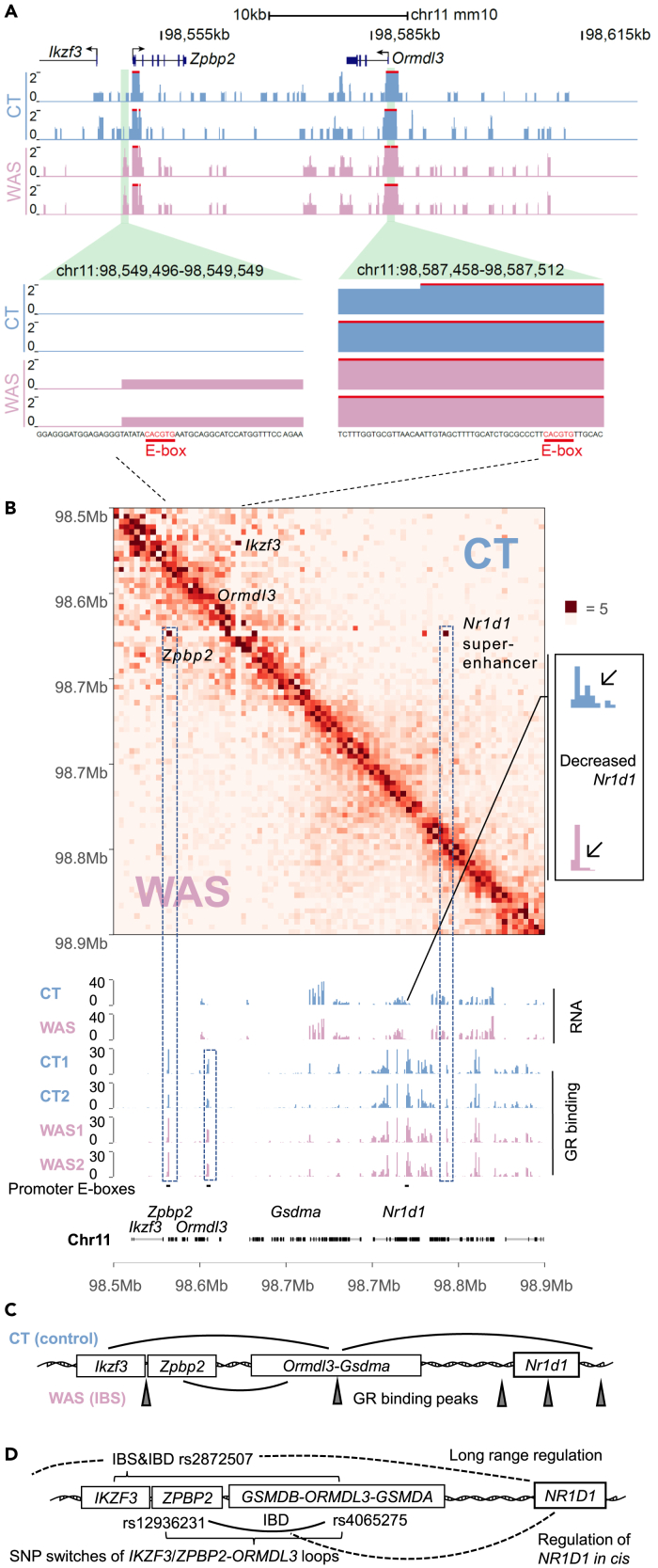
GR remodeled Ikzf3-Nr1d1 circadian chromatin loops between GR-bound E-box sites
Within our conserved target region of interest (Ikzf3-Nr1d1), bidirectional Ikzf3(3′–5′)-Zpbp2 (5′–3′), Ormdl3, and Nr1d1 promoters have conserved E-boxes. GR binding at these promoters and the WAS-induced Ikzf3-Zpbp2 promoter E-box GR peak suggest long-range regulation in addition to the promoter activity (Figure 4A).35 To clarify the effect of stress on chromatin 3D structures, we employed a high-accuracy BL-Hi-C method.48 Furthermore, we compared the chromatin 3D structures with transcriptome and GR cistrome data. In this region, we found that the WAS-induced Zpbp2-Ormdl3 chromatin loop replaced the Ikzf3-Ormdl3 and Ormdl3-Nr1d1 super-enhancer loops in control at the chromatin looping sites that include the GR bound Ikzf3-Zpbp2 bidirectional promoter and Nr1d1 super-enhancer.22,35 In addition, WAS elevated Nr1d1 super-enhancer GR binding (Figure 4B). This pattern correlates with IBS and IBD SNP rs2872507 regulates transcription of this region and IBD SNP (rs12936231) mediated long-range regulation of NR1D1 via the formation of IBD-related ZPBP2-ORMDL3 loop (Figures 4C and 4D).27,35,36,37 Moreover, WAS-induced 5′ distal Fbxl20-Ormdl3 and Ppp1r1b-Ormdl3 loops between the circadian Nr1d1 super-enhancer interactome sites replaced the Stard3-Ormdl3 loop in control (Figure S4).22,42 These data suggest that GR reprogrammed Ikzf3-Nr1d1 circadian controlled chromatin in response to the stress, formation of conserved pathological Zpbp2-Ormdl3 loop abolished Ormdl3-Nr1d1 super-enhancer loop, which participated Nr1d1 circadian transcription.22
Figure 4.
Stress-induced Ikzf3-Zpbp2-Ormdl3-Nr1d1 circadian chromatin loop misalignment
(A and B) BL-Hi-C was performed with colon IECs isolated from two control (CT) and two water avoidance stressed (WAS) BALB/c mice; data from each group were combined for visualization; each RNA track represents the combined RNA-seq data from three mice; GR-CUT&Tag tracks are also shown.
(C) The stress-induced Zpbp2-Ormdl3 loop replaced the Ikzf3-Ormdl3 & Ormdl3-Nr1d1 super-enhancer in control.
(D) This region is conserved with human IKZF3-ZPBP2-ORMDL3-NR1D1 chromatin, with disease-risk SNPs causing switching of the IKZF3-ORMDL3/ZPBP2-ORMDL3 chromatin loops to mediate long-range regulation of NR1D1 transcription.
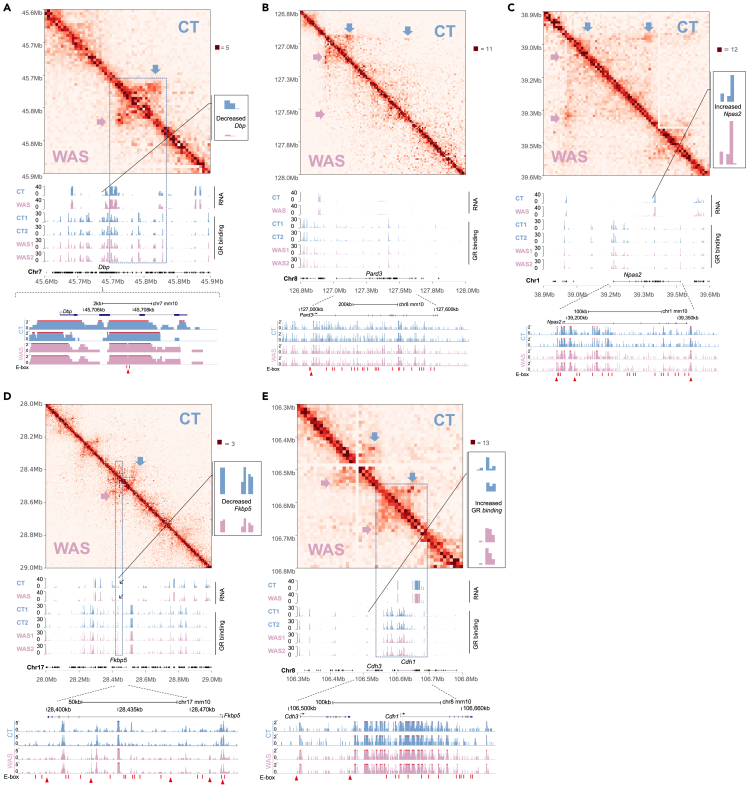
Stress altered circadian-regulated, GR-regulated, and IBS-related chromatin 3D structures
In addition to Nr1d1, WAS also altered other circadian-regulated 3D chromatin architectures, including the clock gene Dbp, the circadian chromatin hub gene Pard3, and the Nr1d1/Rev-erbα regulated clock gene Npas2 (Figures 5A–5C).15 Dbp locus displayed increased chromatin clustering close to the Dbp gene, correlating with decreased transcription (Figure 5A).8,10,14 In addition, we observed WAS-induced Pard3 chromatin loop changes correlating with GR binding (Figure 5B).15,41 Stress also altered NR1D1-regulated Npas2 circadian chromatin loops and increased Npas2 promoter GR binding relevant to increased Npas2 expression (Figure 5C).16,21 These data further elucidated the 3D chromatin structures of GR-mediated circadian misalignment in addition to the stress-disturbed Ikzf3-Nr1d1 chromatin looping, which disturbed the circadian transcription network (Figures 1G and 4).2,10,14 Furthermore, we analyzed stress-transformed 3D chromatin architectures of GR target genes. IBS-related Fkbp5 exhibited a stress-elevated stripe-like structure that correlated with GR binding peak and decreased transcription, similar to GR-NIPBL mediated long-range regulation (Figure 5D).13,49 IBS DEG CDH3 3D structure is likely involved in stress-impaired colon homeostasis.29,33 We observed stress-induced GR binding changes around Cdh3 and altered Cdh3-Cdh1 chromatin structure targeted by GR that were consistent with the transcription pattern (Figures 1E and 5E). These chromatin 3D structure changes suggest the existence of stress-GR transcriptional programming in IBS at multiple conserved genomic loci.2
Figure 5.
Stress altered circadian-regulated, GR-regulated, and IBS-related chromatin 3D structures
BL-Hi-C was performed with colon IECs isolated from two control (CT) and two water avoidance stressed (WAS) BALB/c mice; data from each group were combined for visualization; each RNA track represents the combined RNA-seq data from three mice; GR-CUT&Tag tracks are also shown. E-boxes (CACGTG) and differential GR binding are marked.
(A) Circadian gene Dbp chromatin.
(B) Circadian chromatin hub Pard3.
(C) The NR1D1 target circadian gene Npas2.
(D) GR target gene Fkbp5 stripe-like structure.
(E) IBS-related Cdh3 (P-cadherin)-Cdh1 (E-cadherin) chromatin.
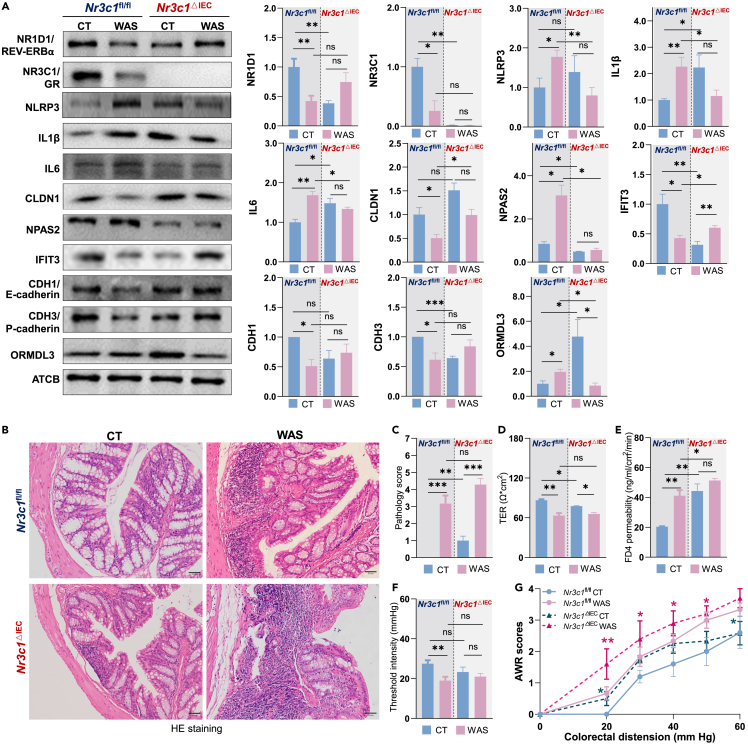
Stress-induced GR transcriptional regulation is validated by intestinal deletion of Nr3c1
Having established the role of GR in transcriptional regulation of the IECs, we next validated this in an in vivo model by generating BALB/c Nr3c1▵IEC conditional knockout mice according to the protocol used with C57BL/6J background.50 In the PC analysis of RNA-seq, Nr3c1▵IEC mice exhibited a closer correlation between CT and WAS groups, with fewer DEGs in the volcano plot and altered KEGG pathway enrichment (Figures 6A–6C). Within the TF DEGs, Nr3c1 deletion changed the expression of steroid-responding transcription factors, including decreased Nr3c1 levels and upregulation of the estrogen receptors Esr1 and Esrrg. Stress elevated Esr1 and Esrrg mRNA levels in Nr3c1fl/fl, but not in Nr3c1▵IEC mice. WAS elevated Egr1 in Nr3c1fl/fl mice. IBS-related TF genes Nfkbiz, Irf7, and Prdm1 were detected as shared TF DEGs of stressed Nr3c1fl/fl and Nr3c1▵IEC mice; Ikzf1 and Ikzf3 were detected as WAS-induced DEG in Nr3c1▵IEC mice.27,28,31,51 Nr3c1 is the only TF DEG between stressed Nr3c1fl/fl and Nr3c1▵IEC mice, supporting GR as the key regulator of the stress-modulated transcription network (Figures 1F, 1G, and 7D). In accordance with the predicted transcription regulatory network, the deletion of Nr3c1 altered the transcription of the circadian TF genes Per2, Per3, Id2, Tef, Arntl/Bmal1, Dbp, and Rorc (Figures 1G, 6D, and 6E). This pattern and GR binding along these TF genes supported the pivotal role of GR in stress-induced transcriptional re-programming, which involves the circadian transcription network (Figures 1F, 1G, and S5).2 In our hypothesis-driven analysis, Nr3c2 (mineral corticoid receptor/MR, GC’s alternative receptor) was upregulated in Nr3c1▵IEC mice as potential compensation for GR loss. Furthermore, chronic stress repressed Nr3c2 in Nr3c1▵IEC mice (Figure 6E). Notably, the deletion of Nr3c1 partially reversed the wild-type (WT) DEG pattern of the Nr1d1-Ikzf3-Grb7-Erbb2 chromatin that we had observed in the WT mice (Figures 1E, 4, 6E, and S4).
Figure 6.
Intestinal Nr3c1 deletion altered stress-induced transcriptomic changes
Control (CT) and water avoidance stressed (WAS) Nr3c1fl/fl and Nr3c1△IEC BALB/c mouse colon epithelium cells were isolated for RNA-seq analysis.
(A) Principal component analysis.
(B) Volcano plot of DEGs.
(C) Intestinal deletion of Nr3c1 in BALB/c altered KEGG pathway enrichment of WAS-induced DEGs.
(D) Transcription factor DEGs.
(E) Heatmap of steroid responding, circadian, GR target, and IBS-related DEGs. Statistical significance was determined using an unpaired t test with Welch’s correction (∗, p < 0.05; ∗∗, p < 0.01; ∗∗∗, p < 0.001; N = 3).
Figure 7.
Intestinal Nr3c1 deletion supported GR’s transcriptional regulation of colonic homeostasis
(A) Western blotting analysis of Nr3c1fl/fl and Nr3c1△IEC BALB/c colon IECs from CT (control) and water avoidance stressed (WAS) groups. Nr3c1 deletion tended to reverse the WAS-induced changes in protein levels. Nr3c1 deletion also reduced the significance of stress-induced changes.
(B) Typical hematoxylin-eosin (HE) staining of CT and WAS colon epithelium from Nr3c1fl/fl and Nr3c1△IEC mice.
(C) Histological scores. Nr3c1 deletion elevated inflammatory infiltration, while stress enhanced the severity.
(D and E) Nr3c1 deletion impaired barrier function and reduced the significance of stress-increased permeability; TEER (transepithelial electrical resistance) and FD4 (fluorescein isothiocyanate-dextran 4 kDa) permeability were measured.
(F) Stress reduced the thresholds of pain responses in Nr3c1fl/fl mice but not in Nr3c1△IEC mice. Data are expressed as means ± standard error. Statistical significance was determined using an unpaired t test with Welch’s correction (∗, p < 0.05; ∗∗, p < 0.01; ∗∗∗, p < 0.001; N = 3, 4, 5).
(G) Nr3c1 is involved in stress-induced visceral hyperalgesia. AWR (abdominal withdrawal reflex) scores in response to CRD (colorectal distension) were measured. AWR data were analyzed with two-way ANOVA analysis (with Tukey’s multiple comparison test); the significance between Nr3c1△IEC WAS/Nr3c1fl/fl CT and Nr3c1△IEC WAS/Nr3c1△IEC CT is illustrated (∗, p < 0.05; ∗∗, p < 0.01; N = 6).
Western blotting revealed that Nr3c1 deletion tended to reverse the WAS-induced changes in NR1D1, NLRP3, IL1β, CDH1, CDH3, and ORMDL3 protein levels (Figure 7A). Nr3c1 knockout also reduced the significance of stress-induced changes in NR1D1, NLRP3, IL1β, IL6, CLDN1, NPAS2, CDH1, and CDH3 expression, which are responsible for impaired intestinal homeostasis (Figures 2A and 7A).5,16 The stress-GR-NR1D1-NLRP3 inflammasome pathway is further confirmed (Figures 2, 3, and 7).2,16 Deletion of Nr3c1 in BALB/c impaired the colon epithelium barrier function and homeostasis, similar to the effects observed on the C57BL/6J background (Figures 7B–7E).50 WAS caused less permeability change in the Nr3c1▵IEC mice than in Nr3c1fl/fl mice, correlating with Nr3c1 deletion prevented WAS-induced barrier function protein CLDN1, CDH1, and CDH3 decreases (Figures 7A–7E). BALB/c Nr3c1fl/fl mice had lower pain threshold than WT mice, possibly due to the side effects of tamoxifen injections, which cause pain (Figures 2E and 7F). Although two-way ANOVA analysis did not detect significant differences between the Nr3c1fl/fl CT and WAS mice, significantly increased pain responses were detected between Nr3c1fl/fl CT/Nr3c1▵IEC WAS and Nr3c1▵IEC CT/Nr3c1▵IEC WAS groups (Figures 7F and 7G). These results correlated with the stress-induced “vascular smooth muscle contraction” pathway changes detected in Nr3c1fl/fl mice regulated by GR binding (Figures 3C and 6C). These protein level changes correlating with IBS phenotypes supported GR’s transcriptional programming in stress-induced IBS (Figures 6 and 7).2,50 We compared published human IBS transcriptome, GWAS, and 3D genomic GBA mapping data with animal dataset generated in this study and found a potential conserved GR mediated Ikzf3-Nr1d1 chromatin circadian misalignment responsible for IBS phenotypes of colonic inflammation and increased permeability and visceral hyperalgesia (Figure S6).
Discussion
In this study, we provided the first evidence of chromatin 3D structure changes in the stress-induced IBS animal model, a significant advance from previous knowledge of epigenetics.14,32,52 This proof-of-concept study suggests that this novel GR-mediated circadian-stress crosstalk in the 3D genome contributes to the IBS-related transcription changes, especially at the conserved Ikzf3-Nr1d1 chromatin regulated by TFs GR, BMAL1, and NR1D1, and chromatin regulators HDAC3, cohesin, and CTCF (Figure S6).13,22,38 Circadian programming of this Nr1d1 chromatin by chromatin remodeler HDAC3 controls diurnal rhythms of the host intestinal metabolism in a microbiota-dependent manner.38 Alteration in Nr1d1 transcription at ZT0 may cause downstream increased Cry1 transcription, which is repressed by NR1D1-mediated enhancer-promoter looping; these findings supported our original hypothesis (Figure 1 and S2).2,21 It can be speculated that prolonged WAS-induced circadian misalignment may contribute to dysbiosis and colitis.14,20,53 These mouse model findings consistent with human data suggest a conserved synergy of GR, BMAL1, HDAC3, and cohesin-CTCF regulate IKZF3-NR1D1 chromatin looping7,13,14,18,19,22 (Figure S6B). Since this chromatin region is epigenetically programmed by intestinal microbiota, personalized probiotics based on IKZF3-NR1D1 SNPs and differential IKZF3 levels in subtypes of IBS could be a potential intervention against the pathological chromatin circadian misalignment31,35,38 (Figure 4D). The potential stress-GR-NR1D1-NLRP3 inflammasome IBS pathology pathway identified in this study could be intervened by the circadian hormone melatonin treatment.40,54,55 Barrier function gene PARD3 has IBS SNPs.45,56 PARD3 is positioned diurnally to the H3K9me2 repressive nuclear lamina-associated domains in human colon epithelial HCT116 cells.41,45 We detected Pard3 promoter methylation and repressed transcription in our parallel WAS rat study.57 Mouse data in this study provided further evidence suggesting the stress-disturbed circadian regulation of Pard3 (Figure 5B). The IBS-related CDH3(P-cadherin)-CDH1(E-cadherin) chromatin encodes genes responsible for colon barrier function and homeostasis.9,27,29,33 Cdh3 and Cdh1 are also WAS-induced DEGs in our parallel rat study.58 Our mouse data unveiled stress-induced remodeling of this chromatin region in 3D (Figure 5E). We detected multiple IBS-related TF DEGs in the Nr3c1 knockout mice, including Egr1, Nfkbiz, Irf7, Prdm1, Ikzf1, and Ikzf3; suggesting the GR-regulated colon IEC transcription network responsible for IBS (Figure 6).23,27,28,31,51 Human EGR1 (NGFIA) is responsible for stress-induced NR3C1 promoter DNA methylation and regulation of IBS-related serotonin synthesis; significant differential expression of EGR1 is observed in IBS patient biopsies.4,31,59,60 Mathematicians studied the circadian 4DN to model genomic programming of tissue homeostasis, leading to the proposal of the two-gene (mutual TF inhibition) network “hardwiring.”61 Based on our previous study, we proposed the formation of GR-HES1 pair in the colon crypt axis, and HES1 was identified as a decreased IBS DEG (IBS-C vs. Healthy Control, p < 0.0001; IBS-D vs. Healthy Control, p < 0.01).1,5,29,62 The current study provided evidence of GR-NR1D1 hardwiring in the circadian time axis.61,62 The colon homeostasis is likely programmed by GR coupled “hardwiring” in time and space.1,2,61 Finally, these findings support the dynamic synergy between TFs and chromatin looping machinery in stress-GC triggered genomic programming with SNP-determined differences between individuals (Figure S6B).2,13,20,22
Information provided in this study has potential applications for developing innovative precision therapy strategies for human bowel disorders (Figures S1 and S6).2,23,32 BALB/c and C57BL/6J strains have been extensively compared in multiple in vivo studies targeting the GBA.53,63 Intestinal deletion of Nr3c1 in BALB/c resulted in impaired barrier function previously reported in C57BL/6J; BALB/c Nr3c1⊿IEC mice demonstrated higher stress-induced visceral hyperalgesia than Nr3c1fl/fl mice (Figures 7B–7G). The BALB/c strain complemented the C57BL/6J genetic background in this study of Nr1d1 regulation.63 The BALB/c Nr3c1⊿IEC mice, which were first generated in this study, may be helpful in further explorations of the GR function. In addition, the BALB/c×C57BL/6J mouse model may elucidate the role of allele-specific gene expression in regulating genome architecture in vivo and help develop and validate next-generation pharmacogenomics.2,53,63 We observed transcriptome differences between WT and Nr3c1fl/fl BALB/c mice treated for 5 days with tamoxifen (estrogen analog) before the WAS procedure thus supporting the existence of pharmacological crosstalk that attenuates GC actions. These data indicate that extensive crosstalk between steroids and their nuclear receptors is involved in stress responses (Figures 1F and 6). To represent the full spectrum of patients, datasets generated in females are needed as current datasets are biased toward males. In conclusion, we found GR-mediated Ikzf3-Nr1d1 3D chromatin circadian misalignment in a stress-induced IBS animal model, which impaired the circadian transcription regulatory network responsible for intestinal homeostasis. The GR-regulome dataset generated in this study could benefit the development of precision medicine algorithms based on the conserved transcriptional regulation by GR and transcription regulatory enhancer SNPs responsible for IBS (Figure S6).2,23
Limitations of the study
Pooled colon IECs in generating the proof-of-concept dataset represent a mixture of different epithelial subtypes. More datasets generated in females are needed to represent the full spectrum of patients.
Ethics approval
All experimental procedures were performed following the ethical guidelines of the Animal Management Rules of the Chinese Ministry of Health (Document No.55,2001), and approved by the Animal Care and Use Committee, Union Hospital, Tongji Medical College, HUST, China (Approval ID 2016-0057).
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Glucocorticoid Receptor Rabbit mAb | Cell Signaling Technology | #3660 (D8H2) |
| Anti-NR1D1 antibody | Abcam | ab174309 [EPR10376] |
| NLRP3 Rabbit mAb | Cell Signaling Technology | #15101 (D4D8T) |
| IL1β Rabbit pAb | ABclonal | A16288 |
| IL-6 Rabbit mAb | Cell Signaling Technology | #12912 (D5W4V) |
| Claudin 1 Monoclonal Antibody | Thermo Fisher | 37-4900 (2H10D10) |
| ORMDL3 Rabbit pAb | ABclonal | A14951 |
| NPAS2 Rabbit pAb | ABclonal | A16930 |
| PARD3 Polyclonal antibody | Proteintech | 11085-1-AP |
| β-Actin Rabbit mAb | ABclonal | AC026 |
| ARNTL Polyclonal antibody | Proteintech | 14268-1-AP |
| Caspase 1/p20/p10 Polyclonal antibody | Proteintech | 22915-1-AP |
| IFIT3 Rabbit pAb | ABclonal | A3924 |
| E-Cadherin Rabbit pAb | ABclonal | A11492 |
| CDH3 Rabbit pAb | ABclonal | A14235 |
| Glucocorticoid Receptor Rabbit mAb | Cell Signaling Technology | #12041 (D6H2L) |
| Rev-Erbα Rabbit mAb | Cell Signaling Technology | #13418 (E1Y6D) |
| Chemicals, peptides, and recombinant proteins | ||
| SR9009 | MedChemExpress | HY-16989 |
| Fluorescein isothiocyanate–dextran | Sigma-Aldrich | FD4-1G |
| Insulin | Procell Life Science&Technology | PB180432 |
| Recombinant Murine IFN-γ | PeproTech | 315-05 |
| Corticosterone | MedChemExpress | HY-B1618 |
| Mifepristone | MedChemExpress | HY-13683 |
| RPMI 1640 | Thermo Fisher | 11875093 |
| Fetal Bovine Serum | Thermo Fisher | A4766801 |
| Critical commercial assays | ||
| Dual-Luciferase® Reporter Assay System | Promega | E1910 |
| Deposited data | ||
| IBS colon epithelium transcriptome from water avoidance stressed BALB/c & C57BL/6J mice | https://www.ncbi.nlm.nih.gov/sra | PRJNA792732 |
| GR CUT&Tag analysis of colon epithelium from water avoidance stressed BALB/c mice IBS model | https://www.ncbi.nlm.nih.gov/sra | PRJNA882590 |
| BL-Hi-C analysis of colon epithelium from water avoidance stressed BALB/c mice IBS model | https://submit.ncbi.nlm.nih.gov/geo/ | GSE233649 |
| IBS colon epithelium transcriptome from water avoidance stressed floxed and intestinal GR knockout BALB/c mice | https://www.ncbi.nlm.nih.gov/sra | PRJNA884499 |
| Experimental models: cell lines | ||
| YAMC | Wuhan Union Hospital | |
| Experimental models: organisms/strains | ||
| BALB/c mice | HFK Bioscience | |
| BALB/c-Nr3c1em1(flox)Smoc | Shanghai Model Organisms Center | |
| BALB/c-Vil1em1(2A-CreERT2-SV40poly(A) Smoc | Shanghai Model Organisms Center | |
| Oligonucleotides | ||
| Actb qPCR primer | CATTGCTGACAGGATGCAGAAGG/TGCTGGAAGGTGGACAGTGAGG | |
| Nr1d1qPCR primer | CAGGCTTCCGTGACCTTTCTCA/TAGGTTGTGCGGCTCAGGAACA | |
| Nr1d2qPCR primer | CAGTGAGAAGCTGAATGCCCTC/TGCACGGATGAGTGTTTCCTGC | |
| DbpqPCR primer | ACACCGCTTCTCAGAGGAGGAA/TCTCGACCTCTTGGCTGCTTCA | |
| Cry1qPCR primer | GGTTGCCTGTTTCCTGACTCGT/GACAGCCACATCCAACTTCCAG | |
| Nfil3 qPCR primer | CAGGACTACCAGACATCCAAGG/AGGACACCTCTGACACATCGGA | |
| Npas2qPCR primer | ACAGCACCACAGCTTTGCCAAG/CAGCAGGAGTTGCTTTGTGAGG | |
| ArntlqPCR primer | ACCTCGCAGAATGTCACAGGCA/CTGAACCATCGACTTCGTAGCG | |
| ClockqPCR primer | GGCTGAAAGACGGCGAGAACTT/GTGCTTCCTTGAGACTCACTGTG | |
| Per2qPCR primer | CTGCTTGTTCCAGGCTGTGGAT/CTTCTTGTGGATGGCGAGCATC | |
| Recombinant DNA | ||
| pGL3-Basic | Promega | E1751 |
| Software and algorithms | ||
| Prism 9.0 | GraphPad | |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to lead contact Gen Zheng (zhenggen@hotmail.com).
Materials availability
BALB/c-Nr3c1em1(flox)Smoc and BALB/c-Vil1em1(2A-CreERT2-SV40poly(A) Smoc mice generated in this study will be commercially available from Shanghai Model Organisms Center, Inc.
Experimental model and subject details
Animals
Adult (aged 8 weeks) male BALB/c mice were purchased from HFK Bioscience (Beijing, PRC). All mice were allowed to acclimatize to the animal facility one week after arrival. Mice were group-housed (4/cage) with free access to food and water under a 12:12 h (9 am/9 pm) light-dark cycle in a temperature and humidity-controlled environment. Animals were randomly grouped. Mice were repeatedly exposed to WAS as described previously.4 The mice were placed on 6×6 cm stainless steel platforms in the middle of tanks filled with water (25°C) to 1 cm below the height of the platforms. The animals were maintained in the tank for 1 h in the morning (9 am to 10 am) daily for 10 consecutive days. The control mice were kept in standard cages. 50 mg/kg SR9009 (MedChemExpress) was administered to mice via intraperitoneal injection once daily at ZT8.16
Flox heterozygous mice (Nr3c1flox/+) and Vil1 gene-directed knock-in 2A-CreERT2-polyA heterozygous mice were produced by Shanghai Biomodel Organism Science & Technology Development Co., Ltd. Genomic DNA was isolated from toe biopsies and all offspring were genotyped by PCR. Heterozygous Nr3c1flox/+ mice were mated with Villin-Cre transgenic mice to generate flox and Cre double-positive heterozygous mice (Nr3c1flox/+: Cre+), which were mated with heterozygous Nr3c1flox/+ mice to obtain flox homozygous and Cre-positive experimental mice (Nr3c flox/flox: Cre+) and flox homozygous and Cre-negative control mice (Nr3c1flox/flox), all on a BALB/c background. All mice were injected intraperitoneally with 2 mg tamoxifen (sunflower oil/ethanol mixture, 10:1 v/v) for five consecutive days before the induction of WAS.
Colon epithelium cell isolation
Mice were sacrificed with cervical dislocation after isoflurane anesthesia, and the colons (approximately 0.5–4 cm from the anus/distal and middle colon) were collected, cut open into approximately 5 mm sections, and incubated in Dulbecco's phosphate-buffered saline (DPBS /without Ca2+ & Mg2+) containing 8 mM EDTA and 1 mM DTT in 15-ml tubes. After rotation for 75 min at 4°C, the crypts were released and collected by centrifugation at 50 ×g for 2 min at 4°C. After a brief wash with ice-cold PBS, the crypts were snap-frozen in liquid nitrogen and stored at -80°C before use. The detailed protocol is available on the TTML website (www.umichttml.org).
The ZT0 time-point was used to minimize the effect of ileal-derived GC on the transcriptional network orchestrated by the circadian clock and microbiota. We used acutely harvested enriched colon epithelial cells to generate transcriptome, GR cistrome, and BL-Hi-C datasets to compare with published human data for conserved genomic mechanisms (Figure S1).
RNA-seq analysis
RNA and DNA were isolated with TIANamp DNA RNA Isolation Kit (Tiangen DP422, Beijing, China) following the manufacturer’s instruction and analyzed with Nanodrop (Thermo Fisher) and 2100 Bioanalyzer (Agilent). Biological triplicates were used for every condition. RNA samples were submitted to Wuhan Frasergen Bioinformatics Co., Ltd for RNA-seq analysis.
Library preparation for RNA sequencing. A total amount of 1 μg RNA per sample was used as input material for the RNA sample preparations. Sequencing libraries were generated using NEBNext® UltraTM RNA Library Prep Kit for Illumina® (NEB) following the manufacturer’s recommendations, and index codes were added to attribute sequences to each sample. Briefly, mRNA was purified from total RNA using poly-T oligo-attached magnetic beads. Fragmentation was carried out using divalent cations under elevated temperature in NEB Next First Strand Synthesis Reaction Buffer. First-strand cDNA was synthesized using random hexamer primers and M-MuLV Reverse Transcriptase (RNaseH-). Second-strand cDNA synthesis was subsequently performed using DNA Polymerase I and RNase H. Remaining overhangs were converted into blunt ends via exonuclease/polymerase activities. After adenylation of 3' ends of DNA fragments, NEB Next Adaptor with hairpin loop structure was ligated to prepare for hybridization. In order to select cDNA fragments with the right length, the library fragments were purified with the AMPure XP system (Beckman Coulter, Beverly, USA). Then 3 μl uracil-specific excision reagent (USER) Enzyme (NEB) was used with size-selected, adaptor-ligated cDNA at 37°C for 15 min followed by 5 min at 95°C before PCR. Then PCR was performed with Phusion High-Fidelity DNA polymerase, Universal PCR primers, and Index (X) Primer. At last, products were purified (AMPure XP system), and library quality was assessed on the Agilent Bioanalyzer 2100 System.
Clustering and sequencing. The clustering of the index-coded samples was performed on a cBot Cluster Generation System using HiSeq X Ten Cluster Kit (Illumina), following the manufacturer’s instructions. After cluster generation, the library preparations were sequenced on an Illumina Nova 6000 platform, and 150 bp paired-end reads were generated.
Sequencing data analysis. Before doing any further analysis, quality control was performed. Software FraserQC (v1.2) was used to do quality control. FraserQC is an in-house software developed by Frasergen Co.Ltd, and analyzed in default parameters. Align reads to the reference genome; we took the mouse mm10 genome as the reference genome for this project. Sequencing reads were aligned to the reference genome using Tophat2(v2.1.1), Tophat2(v2.1.1) and bowtie2(v2.2.2) in and bowtie2(v2.2.2) in default parameter. Genes and isoforms expression levels are quantified by a software package: RSEM (RNASeq by Expectation-Maximization v1.3.0). RSEM computes maximum likelihood abundance estimates using the Expectation-Maximization (EM) algorithm as its statistical model.
EdgeR (v3.6.8) package method was used for screening differentially expressed genes. EdgeR implements a statistical methodology based on the negative binomial distribution, including empirical Bayes estimation, exact tests, generalized linear models, and quasi-likelihood tests. We screen differentially expressed genes according to the following criteria: Foldchange ≥2 and FDR <0.05. KOBAS (v3.0) was used to do pathway enrichment analysis of DEGs. KOBAS 3.0 is a server for gene/protein functional annotation (Annotate module) and functional gene set enrichment (Enrichment module). The Annotate module accepts gene lists as input, including IDs or sequences, and generates annotations for each gene based on multiple databases of pathways, diseases, and Gene Ontology. The Enrichment module accepts either gene list or gene expression data as input and generates enriched pathways, corresponding gene names, P-value of enrichment, and enrichment score. Transcription regulators were predicted using the web server BART (Binding Analysis for Regulation of Transcription http://bartweb.org/ version 2.0) with all the DEGs detected above. The top 50 transcription regulators identified with BART were used for predicting the transcription network with the Cytoscape web server (https://cytoscape.org/).
qPCR analysis
qPCR analysis was performed with HiScript III room temperature (RT) SuperMix for qPCR (#R323) and AceQ qPCR SYBR Green Master Mix (#Q111) from Vazyme, PRC. qPCR primer sequences from https://www.origene.com/category/gene-expression/qpcr-primer-pairs were used.
Western Blotting
For immunoblot analysis, isolated colon crypts or YAMC cells scraped off from the dish were lysed with NP40 lysis buffer (50 mmol/L Tris-HCl, 150 mmol/L NaCl, 1% NP40, pH 8.0) supplied with protease inhibitor cocktail (Roche, USA) on ice for 20 min before 5 min 6000 rpm centrifugation at 4°C. Supernatants were collected for SDS-PAGE analysis. The primary antibodies were incubated overnight at 4°C.
Antibodies: GR/NR3C1 (Cell Signaling Technology, Cat.3660, 1:1000), REV-ERBα /NR1D1(Abcam, Cat. ab174309, 1:1000), NLRP3(Cell Signaling Technology, Cat.15101, 1:1000), IL1β(Abclonal, Cat.A16288, 1:1000), IL6 (Cell Signaling Technology, Cat.12912, 1:1000), CLDN1 (Thermo Fisher, Cat.37-4900, 1:10000), ORMDL3 (Abclonal, Cat.A14951, 1:2000), NPAS2 (Abclonal, Cat.A16930, 1:2000), PARD3 (Proteintech, Cat.11085-1-AP, 1:500), ACTB/β-actin (Abclonal, Cat.AC026, 1:10000), BMAL1 (Proteintech, Cat.14268-1-AP, 1:5000), CLOCK (Abclonal, Cat.A7265, 1:10000), Caspase-1 (Proteintech, Cat.22915-1-AP, 1:1000), IFIT3 (Abclonal, Cat.A3924, 1:1500), CDH1 (Abclonal, Cat.A11492, 1:1500), CDH3 (Abclonal, Cat.A14235, 1:1500).
Histological analysis
For histological analysis, distal colon specimens were fixed in 4% formalin for 24 h and embedded in paraffin, stained with hematoxylin and eosin (H&E). Analysis was then performed by a pathologist blinded to the experimental groupings. The sections were graded as inflammation, crypt injury, and ulceration, as shown in Tab S1; scores were calculated by adding up the score for these grades.
Colon permeability assessment
The colon was removed quickly and flushed with cold Krebs solution (121 mM NaCl, 25 mM NaHCO3, 3.8 mM KCl, 1 mM KH2PO4, 1.2 mM CaCl2, 1.2 mM MgSO4, and 11.1 mM glucose). Then each piece was placed into an Ussing chamber (Physiological Instruments, San Diego, CA). The chambers were filled with 5 ml of Krebs solution, maintained at 37°C, and given oxygen throughout the experiment. After a 30 min equilibration period, the spontaneous potential difference and short-circuit current (Isc) in the Ussing chamber were recorded. The transepithelial resistance was calculated using the Acquire and Analyze 2.3 software. 3 ml of FITC-dextran (FD4, 1 mg/ml, Sigma-Aldrich) was added to the mucosal side, and an equal volume of Krebs solution to the other side of each chamber. 100 μl samples were collected and transferred to 96-well plates in duplicate every 30 min. Each sample was checked for FD4 flux at 520 nm with a fluorescence microplate reader (Molecular Devices, USA). The permeability of each tissue was expressed as the calculated flux of FD4 over 30-60 min.
CRD and abdominal withdrawal reflex scoring
We assessed visceral sensitivity by measuring the behavioral response of the AWR to colorectal distension (CRD). During the test, mice were placed in a restraint container, and they could not escape or turn around. Mice were lightly sedated with halothane, and then a soft latex balloon (1.5 cm long, 1 cm diameter) was inserted into the descending colon 1 cm within the anus. The balloon was secured by tying additional tubing to the mouse tail. The mice were accustomed to this procedure 1 day before the experiment to minimize stress. Firstly, the colorectal balloon was gradually inflated by 5 mm Hg until pain manifested to measure threshold intensity. Then, the balloon was rapidly inflated to constant pressure (20, 30, 40, 50, and 60 mm Hg) to measure the AWR. Two blinded observers observed and measured, and we performed three replicates. The AWR scores were graded on a scale of 0-4: 0, no behavioral response to CRD; 1, brief head movement followed by immobility; 2, contraction of abdominal muscles; 3, lifting of abdomen; 4, body arching and lifting of pelvic structures.46
Open-field test
The test mouse was gently placed near the wall side with a length of 50 cm, a width of 50 cm, and a height of 50 cm of the open-field arena and allowed to explore freely for 20 min. The first 10 min were to let the mouse adapt to the environment. Only the last 10 min of the mouse's trajectory was recorded by a video camera and further analyzed with EthoVision XT 13 (Noldus, USA).
CUT&Tag analysis
Equal amounts of colon epithelium cells from four mice were pooled from each group. The CUT&Tag sequencing experiments were performed by Wuhan Frasergen Bioinformatics Co., Ltd. Equal amounts of colon epithelium cells from 4 mice were pooled from each group. Following CUT&Tag analysis was performed by Wuhan Frasergen Bioinformatics Co., Ltd, using an antibody against GR (Cell Signaling, Cat.12041). Isolated colon crypts were fixed with 0.8% formaldehyde in DMEM and quenched with 0.125 M Glycine; then, the nuclei were isolated with 10 mM Tris-HCl pH 8.0, 10 mM NaCl, 0.2% Igepal CA630, protease inhibitors cocktail on ice with 0.5 ml glass grinder. In brief, 10,000-500,000 nuclei were harvested and centrifuged for 3 min at 600×g at RT. Nuclei were washed twice in 100-500 μL Wash Buffer (20 mM HEPES pH 7.5; 150 mM NaCl; 0.5 mM Spermidine; 1× Protease inhibitor cocktail) by gentle pipetting. 10 μL of activated concanavalin A-coated magnetic beads were added per sample and incubated at RT for 10 min. The unbound supernatant was removed, and bead-bound nuclei were resuspended in 50 μL Dig-wash Buffer (20 mM HEPES pH 7.5; 150 mM NaCl; 0.5 mM Spermidine; 1× Protease inhibitor cocktail; 0.05% Digitonin) containing 2 mM EDTA and a 1:50 dilution of the appropriate primary antibody. Primary antibody incubation was performed on a rotating platform for 2 h at RT. Then the liquid was removed from the magnet stand. An appropriate secondary antibody was diluted 1:50 in 50 μL of Dig-Wash buffer, and nuclei were incubated at RT for 1 h. Nuclei were washed using the magnet stand twice for 1 min in 500 μL Dig-Wash buffer to remove unbound antibodies. A 1:200 dilution of pA-Tn5 adapter complex (∼0.04 μM) was prepared in Dig-300 Buffer (0.01% Digitonin, 20 mM HEPES, pH 7.5, 300 mM NaCl, 0.5 mM Spermidine, 1× Protease inhibitor cocktail). After removing the liquid, 100 μL was added to the nuclei with gentle vortexing, and incubated with pA-Tn5 at RT for 1 h. Nuclei were washed twice for 1 min in 500 μL Dig-300 Buffer to remove unbound pA-Tn5 protein. Next, nuclei were resuspended in 300 μL tagmentation buffer (10 mM MgCl2 in Dig-300 Buffer) and incubated at 37°C for 1 h. To stop tagmentation, 10 μL of 0.5 M EDTA, 3 μL of 10% SDS, and 2.5 μL of 20 mg/mL Proteinase K were added to 300 μL of the sample, which was incubated at 55°C for 1 h, purified using phenol-chloroform–isoamyl alcohol and ethanol, washed with 100% ethanol and suspended in water. The DNA was amplified by PCR reaction as follows: 3 min at 72°C and 30 s at 98°C followed by 14-20 cycles of 15 s at 98°C and 30 s at 60°C and 30 s at 72°C, with a final extension at 72°C for 3 min. Finally, the amplified DNA was purified using Ampure XP beads (Beckman). Libraries were sequenced on the Illumina Nova-seq platform.
The CUT&Tag pipeline begins with filtering tools (FastQC and Trimmomatic) to remove this interference information, containing adapter sequences, low-quality bases, and undetected bases (indicated by N) in paired-end raw data. After obtaining clean data through quality control, we use Bowtie2 to map clean reads to the reference genome, and further screen out the low-quality mapping, PCR redundancy, and organelle alignment by Samtools and Picard, and thus get the retained valid pairs for subsequent analysis. Meanwhile, CUT&Tag fragment length distribution is an important index for experiment quality. For this reason, the histograms of the size of the insert are plotted with an R script to check the characteristic peaks, and the results are expected to be like a "wave-shape". Next, we choose MACS2 software based on statistical methods to perform Peak Calling, where the peaks called are significantly enriched regions from retained valid pairs data. For the sequencing experiments with biological replication, the next step is focusing on the reproducibility of the peaks; we compare the two replicate samples to show the overlap between peaks and further evaluate the consistency of peak enrichment multiples. The result of the latter is obtained by a more stringent approach called IDR (Irreproducible Discovery Rate), which is based on the presence or absence of Overlap Peak and considers the consistency of the order of peak enrichment multiples between the two sets of data. After that, the R scripts with Deeptools software are performed to illustrate the signal enrichment analysis of peak regions, gene body regions, and the regions from the TSS (TSS); we also use ChIPseeker to obtain gene annotations on peak regions at the same time. Finally, we visualized the called peaks of all samples using the Integrative Genomic Viewer.
Bisulfite pyrosequencing
Bisulfite pyrosequencing of Nr1d1 TSS E-box was performed by Sangon Biotech (Shanghai, PRC) Co., Ltd.
Cell culture and Luciferase assay
YAMC cells were grown in RPMI 1640 medium containing 5% FBS (Thermo Fisher, USA), 50 μg/ml gentamicin, 1 μg/ml insulin (Procell), and 0.5 ng/ml murine IFNγ (Peprotech) at 33°C, cells with 95-100% confluency were cultured under the nonpermissive condition (37°C without IFN-ϒ) for 24 h differentiation before treatments. Cells were harvested for western blotting analysis after treatment with 1 μM corticosterone (CORT) (MedChemExpress), 500 nM RU486 (Sigma, USA), 10 μM SR9009 (MedChemExpress), and Vehicle. 3Kb N1d1 promoter-luciferase plasmids were generated according to published.16 Mutation of Nr1d1 TSS E-box (CACGTG) was conducted with primer: CCTAGCAAACGTGAGAGCTTAGATTGATTGGAGAACTGACCTCACC, together with its reverse complement. YAMC cells grown at 33°C with 5 U/ml IFN-ϒ with 50% confluency were transfected with plasmids and siRNA with Lipofectamine 3000 (Thermo Fisher) according to the manufacturer's direction, and cells were cultured under the nonpermissive condition 24 h after transfection. Then the transfected cells were harvested for luciferase assay before/after the treatments. Promega (USA) Dual-Luciferase® Reporter system was used to measure the promoter activity.
BL-Hi-C analysis
BL-Hi-C data were generated from colon IEC isolated from two Control (CT) and two WAS model mice. Crosslinked colon epithelium crypts from each mouse were ground in 500 μl nucleus lysis buffer (10 mM Tris-HCl pH 8.0, 10 mM NaCl, 0.2% Igepal CA630, Roche protease inhibitors cocktail) on ice with 0.5 ml glass grinder, and the nuclei were filtered with 40 μm Falcon cell strainer. Then the BL-Hi-C analysis was performed as described previously.48 Correlations among the four libraries were analyzed with hicrep (https://github.com/TaoYang-dev/hicrep) & hicLibRepeatCor (https://gitlab.com/seqyuan/ctools/-/tree/master/module/CQC/libCor) (Figure S7 and TableS2–S5). Data from each group were combined to achieve higher Hi-C resolution in the subsequent analysis. BL-Hi-C data were visualized with Juicebox. Hi-C figures were generated with Python by Frasergen Bioinformatics Co., Ltd, Wuhan.
Statistical analysis
GraphPad Prism 9.0 was used for generating figures and statistical analysis. In our hypothesis-driven analysis, the unpaired t test with Welch’s correction (psychologists’ recommended default) was used to examine the mRNA, protein, and luciferase activity quantification data between the 2 groups. Circadian clock gene expression from multiple time points was analyzed with Mann–Whitney test. AWR data were analyzed with two-way ANOVA analysis with Tukey’s multiple comparisons. Results were expressed as means ± standard error. P<0.05 was considered statistically significant.
Acknowledgments
This study was supported in part by the National Natural Science Foundation of China (Nos. 82070556, 81770539, 81974068, 81900580). The authors thank Bin Zhang, Hongbo Yang, Fang Hua, and Pingfeng Zhang for insightful discussions. The authors thank Lin Liu and Chenkui Kuang for processing the data.
Author contributions
G.Z., J.W., G.H., R.L., and S.T.Z. conceptualized this study. G.Z., S.Y.P., J.B.W., and F.Y.W. performed animal studies. S.Y.P. validated the animal studies. G.Z., Y.C., D.J.X., M.D.J., Q.W., and L.L.Y. performed BL-Hi-C analysis. D.J.X., J.B.W., and M.D.J. visualized omics data. X.H.H., R.L., and Y.Z. supervised this study. G.Z. wrote the original draft. R.L., J.W., G.H., and Y.Z. reviewed &and edited this manuscript.
Declaration of interests
The authors declare that they have no competing interests.
Published: June 17, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.107137.
Supplemental information
Data and code availability
Source of the RNA-seq and CUT&Tag data can be accessed from NCBI SRA (https://www.ncbi.nlm.nih.gov/sra) accession PRJNA792732, PRJNA882590, and PRJNA884499; Hi-C data are available from NCBI GEO (https://www.ncbi.nlm.nih.gov/geo/) accession GSE233649.
References
- 1.Mukherji A., Kobiita A., Ye T., Chambon P. Homeostasis in intestinal epithelium is orchestrated by the circadian clock and microbiota cues transduced by TLRs. Cell. 2013;153:812–827. doi: 10.1016/j.cell.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 2.Wiley J.W., Higgins G.A., Athey B.D. Stress and glucocorticoid receptor transcriptional programming in time and space: Implications for the brain-gut axis. Neuro Gastroenterol. Motil. 2016;28:12–25. doi: 10.1111/nmo.12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu W.L., Adame M.D., Liou C.W., Barlow J.T., Lai T.T., Sharon G., Schretter C.E., Needham B.D., Wang M.I., Tang W., et al. Microbiota regulate social behaviour via stress response neurons in the brain. Nature. 2021;595:409–414. doi: 10.1038/s41586-021-03669-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hong S., Zheng G., Wiley J.W. Epigenetic regulation of genes that modulate chronic stress-induced visceral pain in the peripheral nervous system. Gastroenterology. 2015;148:148–157.e7. doi: 10.1053/j.gastro.2014.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng G., Victor Fon G., Meixner W., Creekmore A., Zong Y., K Dame M., Colacino J., Dedhia P.H., Hong S., Wiley J.W. Chronic stress and intestinal barrier dysfunction: Glucocorticoid receptor and transcription repressor HES1 regulate tight junction protein Claudin-1 promoter. Sci. Rep. 2017;7:4502. doi: 10.1038/s41598-017-04755-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swan C., Duroudier N.P., Campbell E., Zaitoun A., Hastings M., Dukes G.E., Cox J., Kelly F.M., Wilde J., Lennon M.G., et al. Identifying and testing candidate genetic polymorphisms in the irritable bowel syndrome (IBS): association with TNFSF15 and TNFalpha. Gut. 2013;62:985–994. doi: 10.1136/gutjnl-2011-301213. [DOI] [PubMed] [Google Scholar]
- 7.Murayama Y., Yahagi N., Takeuchi Y., Aita Y., Mehrazad Saber Z., Wada N., Li E., Piao X., Sawada Y., Shikama A., et al. Glucocorticoid receptor suppresses gene expression of Rev-erbalpha (Nr1d1) through interaction with the CLOCK complex. FEBS Lett. 2019;593:423–432. doi: 10.1002/1873-3468.13328. [DOI] [PubMed] [Google Scholar]
- 8.Aguilar-Arnal L., Hakim O., Patel V.R., Baldi P., Hager G.L., Sassone-Corsi P. Cycles in spatial and temporal chromosomal organization driven by the circadian clock. Nat. Struct. Mol. Biol. 2013;20:1206–1213. doi: 10.1038/nsmb.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stokes K., Cooke A., Chang H., Weaver D.R., Breault D.T., Karpowicz P. The Circadian Clock Gene BMAL1 Coordinates Intestinal Regeneration. Cell. Mol. Gastroenterol. Hepatol. 2017;4:95–114. doi: 10.1016/j.jcmgh.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thaiss C.A., Levy M., Korem T., Dohnalová L., Shapiro H., Jaitin D.A., David E., Winter D.R., Gury-BenAri M., Tatirovsky E., et al. Microbiota Diurnal Rhythmicity Programs Host Transcriptome Oscillations. Cell. 2016;167:1495–1510.e12. doi: 10.1016/j.cell.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Kim M.J., Choi G.E., Chae C.W., Lim J.R., Jung Y.H., Yoon J.H., Park J.Y., Han H.J. Melatonin-mediated FKBP4 downregulation protects against stress-induced neuronal mitochondria dysfunctions by blocking nuclear translocation of GR. Cell Death Dis. 2023;14:146. doi: 10.1038/s41419-023-05676-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fowler S., Hoedt E.C., Talley N.J., Keely S., Burns G.L. Circadian Rhythms and Melatonin Metabolism in Patients With Disorders of Gut-Brain Interactions. Front. Neurosci. 2022;16 doi: 10.3389/fnins.2022.825246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rinaldi L., Fettweis G., Kim S., Garcia D.A., Fujiwara S., Johnson T.A., Tettey T.T., Ozbun L., Pegoraro G., Puglia M., et al. The glucocorticoid receptor associates with the cohesin loader NIPBL to promote long-range gene regulation. Sci. Adv. 2022;8 doi: 10.1126/sciadv.abj8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pacheco-Bernal I., Becerril-Pérez F., Aguilar-Arnal L. Circadian rhythms in the three-dimensional genome: implications of chromatin interactions for cyclic transcription. Clin. Epigenetics. 2019;11:79. doi: 10.1186/s13148-019-0677-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim Y.H., Lazar M.A. Transcriptional Control of Circadian Rhythms and Metabolism: A Matter of Time and Space. Endocr. Rev. 2020;41:707–732. doi: 10.1210/endrev/bnaa014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang S., Lin Y., Yuan X., Li F., Guo L., Wu B. REV-ERBalpha integrates colon clock with experimental colitis through regulation of NF-kappaB/NLRP3 axis. Nat. Commun. 2018;9:4246. doi: 10.1038/s41467-018-06568-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scuderi S.A., Casili G., Lanza M., Filippone A., Paterniti I., Esposito E., Campolo M. Modulation of NLRP3 Inflammasome Attenuated Inflammatory Response Associated to Diarrhea-Predominant Irritable Bowel Syndrome. Biomedicines. 2020;8 doi: 10.3390/biomedicines8110519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okabe T., Chavan R., Fonseca Costa S.S., Brenna A., Ripperger J.A., Albrecht U. REV-ERBalpha influences the stability and nuclear localization of the glucocorticoid receptor. J. Cell Sci. 2016;129:4143–4154. doi: 10.1242/jcs.190959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caratti G., Iqbal M., Hunter L., Kim D., Wang P., Vonslow R.M., Begley N., Tetley A.J., Woodburn J.L., Pariollaud M., et al. REVERBa couples the circadian clock to hepatic glucocorticoid action. J. Clin. Invest. 2018;128:4454–4471. doi: 10.1172/JCI96138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramamoorthy S., Cidlowski J.A. Ligand-induced repression of the glucocorticoid receptor gene is mediated by an NCoR1 repression complex formed by long-range chromatin interactions with intragenic glucocorticoid response elements. Mol. Cell Biol. 2013;33:1711–1722. doi: 10.1128/MCB.01151-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim Y.H., Marhon S.A., Zhang Y., Steger D.J., Won K.J., Lazar M.A. Rev-erbalpha dynamically modulates chromatin looping to control circadian gene transcription. Science. 2018;359:1274–1277. doi: 10.1126/science.aao6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu Y., Guo W., Li P., Zhang Y., Zhao M., Fan Z., Zhao Z., Yan J. Long-Range Chromosome Interactions Mediated by Cohesin Shape Circadian Gene Expression. PLoS Genet. 2016;12 doi: 10.1371/journal.pgen.1005992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins G.A., Williams A.M., Ade A.S., Alam H.B., Athey B.D. Druggable Transcriptional Networks in the Human Neurogenic Epigenome. Pharmacol. Rev. 2019;71:520–538. doi: 10.1124/pr.119.017681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arloth J., Bogdan R., Weber P., Frishman G., Menke A., Wagner K.V., Balsevich G., Schmidt M.V., Karbalai N., Czamara D., et al. Genetic Differences in the Immediate Transcriptome Response to Stress Predict Risk-Related Brain Function and Psychiatric Disorders. Neuron. 2015;86:1189–1202. doi: 10.1016/j.neuron.2015.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu W., Jiang C., Kim M., Yang W., Zhu K., Guan D., Lv W., Xiao Y., Wilson J.R., Rader D.J., et al. Individual-specific functional epigenomics reveals genetic determinants of adverse metabolic effects of glucocorticoids. Cell Metab. 2021;33:1592–1609.e7. doi: 10.1016/j.cmet.2021.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang L., Di Lorenzo C., Farrugia G., Hamilton F.A., Mawe G.M., Pasricha P.J., Wiley J.W. Functional Bowel Disorders: A Roadmap to Guide the Next Generation of Research. Gastroenterology. 2018;154:723–735. doi: 10.1053/j.gastro.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 27.Camilleri M., Carlson P., McKinzie S., Zucchelli M., D'Amato M., Busciglio I., Burton D., Zinsmeister A.R. Genetic susceptibility to inflammation and colonic transit in lower functional gastrointestinal disorders: preliminary analysis. Neuro Gastroenterol. Motil. 2011;23:935–e398. doi: 10.1111/j.1365-2982.2011.01749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Camilleri M., Carlson P., Acosta A., Busciglio I., Nair A.A., Gibbons S.J., Farrugia G., Klee E.W. RNA sequencing shows transcriptomic changes in rectosigmoid mucosa in patients with irritable bowel syndrome-diarrhea: a pilot case-control study. Am. J. Physiol. Gastrointest. Liver Physiol. 2014;306:G1089–G1098. doi: 10.1152/ajpgi.00068.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mars R.A.T., Yang Y., Ward T., Houtti M., Priya S., Lekatz H.R., Tang X., Sun Z., Kalari K.R., Korem T., et al. Longitudinal Multi-omics Reveals Subset-Specific Mechanisms Underlying Irritable Bowel Syndrome. Cell. 2020;182:1460–1473.e17. doi: 10.1016/j.cell.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eijsbouts C., Zheng T., Kennedy N.A., Bonfiglio F., Anderson C.A., Moutsianas L., Holliday J., Shi J., Shringarpure S., 23andMe Research Team, et al. Genome-wide analysis of 53,400 people with irritable bowel syndrome highlights shared genetic pathways with mood and anxiety disorders. Nat. Genet. 2021;53:1543–1552. doi: 10.1038/s41588-021-00950-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Camilleri M., Magnus Y., Carlson P., Wang X.J., Chedid V., Maselli D., Taylor A., McKinzie S., Kengunte Nagaraj N., Busciglio I., Nair A. Differential mRNA expression in ileal and colonic biopsies in irritable bowel syndrome with diarrhea or constipation. Am. J. Physiol. Gastrointest. Liver Physiol. 2022;323:G88–G101. doi: 10.1152/ajpgi.00063.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Higgins G.A., Hong S., Wiley J.W. The Role of Epigenomic Regulatory Pathways in the Gut-Brain Axis and Visceral Hyperalgesia. Cell. Mol. Neurobiol. 2022;42:361–376. doi: 10.1007/s10571-021-01108-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lasconi C., Pahl M.C., Cousminer D.L., Doege C.A., Chesi A., Hodge K.M., Leonard M.E., Lu S., Johnson M.E., Su C., et al. Variant-to-Gene-Mapping Analyses Reveal a Role for the Hypothalamus in Genetic Susceptibility to Inflammatory Bowel Disease. Cell. Mol. Gastroenterol. Hepatol. 2021;11:667–682. doi: 10.1016/j.jcmgh.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berce V., Kozmus C.E.P., Potočnik U. Association among ORMDL3 gene expression, 17q21 polymorphism and response to treatment with inhaled corticosteroids in children with asthma. Pharmacogenomics J. 2013;13:523–529. doi: 10.1038/tpj.2012.36. [DOI] [PubMed] [Google Scholar]
- 35.Chang M.L., Moussette S., Gamero-Estevez E., Gálvez J.H., Chiwara V., Gupta I.R., Ryan A.K., Naumova A.K. Regulatory interaction between the ZPBP2-ORMDL3/Zpbp2-Ormdl3 region and the circadian clock. PLoS One. 2019;14 doi: 10.1371/journal.pone.0223212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Söderman J., Berglind L., Almer S. Gene Expression-Genotype Analysis Implicates GSDMA, GSDMB, and LRRC3C as Contributors to Inflammatory Bowel Disease Susceptibility. BioMed Res. Int. 2015;2015 doi: 10.1155/2015/834805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmiedel B.J., Seumois G., Samaniego-Castruita D., Cayford J., Schulten V., Chavez L., Ay F., Sette A., Peters B., Vijayanand P. 17q21 asthma-risk variants switch CTCF binding and regulate IL-2 production by T cells. Nat. Commun. 2016;7 doi: 10.1038/ncomms13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuang Z., Wang Y., Li Y., Ye C., Ruhn K.A., Behrendt C.L., Olson E.N., Hooper L.V. The intestinal microbiota programs diurnal rhythms in host metabolism through histone deacetylase 3. Science. 2019;365:1428–1434. doi: 10.1126/science.aaw3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pilz L.K., Quiles C.L., Dallegrave E., Levandovski R., Hidalgo M.P.L., Elisabetsky E. Differential susceptibility of BALB/c, C57BL/6N, and CF1 mice to photoperiod changes. Braz. J. Psychiatry. 2015;37:185–190. doi: 10.1590/1516-4446-2014-1454. [DOI] [PubMed] [Google Scholar]
- 40.Bishehsari F., Keshavarzian A. Microbes help to track time. Science. 2019;365:1379–1380. doi: 10.1126/science.aaz0224. [DOI] [PubMed] [Google Scholar]
- 41.Zhao H., Sifakis E.G., Sumida N., Millán-Ariño L., Scholz B.A., Svensson J.P., Chen X., Ronnegren A.L., Mallet de Lima C.D., Varnoosfaderani F.S., et al. PARP1- and CTCF-Mediated Interactions between Active and Repressed Chromatin at the Lamina Promote Oscillating Transcription. Mol. Cell. 2015;59:984–997. doi: 10.1016/j.molcel.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 42.Furlan-Magaril M., Ando-Kuri M., Arzate-Mejía R.G., Morf J., Cairns J., Román- A., Tenorio-Hernández L., Poot-Hernández A.C., Andrews S., Várnai C., et al. The global and promoter-centric 3D genome organization temporally resolved during a circadian cycle. Genome Biol. 2021;22:162. doi: 10.1186/s13059-021-02374-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Markham A. Tenapanor: First Approval. Drugs. 2019;79:1897–1903. doi: 10.1007/s40265-019-01215-9. [DOI] [PubMed] [Google Scholar]
- 44.Stasi C., Bellini M., Gambaccini D., Duranti E., de Bortoli N., Fani B., Albano E., Russo S., Sudano I., Laffi G., et al. Neuroendocrine Dysregulation in Irritable Bowel Syndrome Patients: A Pilot Study. J. Neurogastroenterol. Motil. 2017;23:428–434. doi: 10.5056/jnm16155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu M., Yang S., Qiu Y., Chen G., Wang W., Xu C., Cai W., Sun L., Xiao W., Yang H. Par-3 modulates intestinal epithelial barrier function through regulating intracellular trafficking of occludin and myosin light chain phosphorylation. J. Gastroenterol. 2015;50:1103–1113. doi: 10.1007/s00535-015-1066-z. [DOI] [PubMed] [Google Scholar]
- 46.Yu Y.B., Zuo X.L., Zhao Q.J., Chen F.X., Yang J., Dong Y.Y., Wang P., Li Y.Q. Brain-derived neurotrophic factor contributes to abdominal pain in irritable bowel syndrome. Gut. 2012;61:685–694. doi: 10.1136/gutjnl-2011-300265. [DOI] [PubMed] [Google Scholar]
- 47.Zimprich A., Garrett L., Deussing J.M., Wotjak C.T., Fuchs H., Gailus-Durner V., de Angelis M.H., Wurst W., Hölter S.M. A robust and reliable non-invasive test for stress responsivity in mice. Front. Behav. Neurosci. 2014;8:125. doi: 10.3389/fnbeh.2014.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liang Z., Li G., Wang Z., Djekidel M.N., Li Y., Qian M.P., Zhang M.Q., Chen Y. BL-Hi-C is an efficient and sensitive approach for capturing structural and regulatory chromatin interactions. Nat. Commun. 2017;8:1622. doi: 10.1038/s41467-017-01754-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith S.B., Maixner W., Palsson O.S., Van Tilburg M.A., Kanazawa M., Whitehead W.E. 584 FKBP5 Gene Is Associated With IBS Diagnosis. Gastroenterology. 2014;146:109. doi: 10.1016/s0016-5085(14)60392-9. [DOI] [Google Scholar]
- 50.Aranda C.J., Arredondo-Amador M., Ocón B., Lavín J.L., Aransay A.M., Martínez-Augustin O., de Medina F.S. Intestinal epithelial deletion of the glucocorticoid receptor NR3C1 alters expression of inflammatory mediators and barrier function. FASEB J. 2019;33:14067–14082. doi: 10.1096/fj.201900404RR. [DOI] [PubMed] [Google Scholar]
- 51.Iribarren C., Nordlander S., Sundin J., Isaksson S., Savolainen O., Törnblom H., Magnusson M.K., Simrén M., Öhman L. Fecal luminal factors from patients with irritable bowel syndrome induce distinct gene expression of colonoids. Neuro Gastroenterol. Motil. 2022;34 doi: 10.1111/nmo.14390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wiley J.W., Chang L. Functional Bowel Disorders. Gastroenterology. 2018;155:1–4. doi: 10.1053/j.gastro.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 53.Watanabe Y., Arase S., Nagaoka N., Kawai M., Matsumoto S. Chronic Psychological Stress Disrupted the Composition of the Murine Colonic Microbiota and Accelerated a Murine Model of Inflammatory Bowel Disease. PLoS One. 2016;11 doi: 10.1371/journal.pone.0150559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Song G.H., Leng P.H., Gwee K.A., Moochhala S.M., Ho K.Y. Melatonin improves abdominal pain in irritable bowel syndrome patients who have sleep disturbances: a randomised, double blind, placebo controlled study. Gut. 2005;54:1402–1407. doi: 10.1136/gut.2004.062034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cai L., Chen Q., Yao Z., Sun Q., Wu L., Ni Y. Glucocorticoid receptors involved in melatonin inhibiting cell apoptosis and NLRP3 inflammasome activation caused by bacterial toxin pyocyanin in colon. Free Radic. Biol. Med. 2021;162:478–489. doi: 10.1016/j.freeradbiomed.2020.11.003. [DOI] [PubMed] [Google Scholar]
- 56.Arredondo-Hernández R., Schmulson M., Orduña P., López-Leal G., Zarate A.M., Alanis-Funes G., Alcaraz L.D., Santiago-Cruz R., Cevallos M.A., Villa A.R., et al. Mucosal Microbiome Profiles Polygenic Irritable Bowel Syndrome in Mestizo Individuals. Front. Cell. Infect. Microbiol. 2020;10:72. doi: 10.3389/fcimb.2020.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu S., Min L., Guo Q., Li H., Yu Y., Zong Y., Wang L., Li P., Gu J., Zhang S. Transcriptome and methylome profiling in a rat model of irritable bowel syndrome induced by stress. Int. J. Mol. Med. 2018;42:2641–2649. doi: 10.3892/ijmm.2018.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wiley J.W., Higgins G.A., Hong S. Chronic psychological stress alters gene expression in rat colon epithelial cells promoting chromatin remodeling, barrier dysfunction and inflammation. PeerJ. 2022;10:e13287. doi: 10.7717/peerj.13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grasberger H., Chang L., Shih W., Presson A.P., Sayuk G.S., Newberry R.D., Karagiannides I., Pothoulakis C., Mayer E., Merchant J.L. Identification of a functional TPH1 polymorphism associated with irritable bowel syndrome bowel habit subtypes. Am. J. Gastroenterol. 2013;108:1766–1774. doi: 10.1038/ajg.2013.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meaney M.J., Szyf M. Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues Clin. Neurosci. 2005;7:103–123. doi: 10.31887/DCNS.2005.7.2/mmeaney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rajapakse I., Smale S. Emergence of function from coordinated cells in a tissue. Proc. Natl. Acad. Sci. USA. 2017;114:1462–1467. doi: 10.1073/pnas.1621145114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zheng G., Kalinin A.A., Dinov I.D., Meixner W., Zhu S., Wiley J.W. Hypothesis: Caco-2 cell rotational 3D mechanogenomic turing patterns have clinical implications to colon crypts. J. Cell Mol. Med. 2018;22:6380–6385. doi: 10.1111/jcmm.13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shimomura K., Kumar V., Koike N., Kim T.K., Chong J., Buhr E.D., Whiteley A.R., Low S.S., Omura C., Fenner D., et al. Usf1, a suppressor of the circadian Clock mutant, reveals the nature of the DNA-binding of the CLOCK:BMAL1 complex in mice. Elife. 2013;2 doi: 10.7554/eLife.00426. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Source of the RNA-seq and CUT&Tag data can be accessed from NCBI SRA (https://www.ncbi.nlm.nih.gov/sra) accession PRJNA792732, PRJNA882590, and PRJNA884499; Hi-C data are available from NCBI GEO (https://www.ncbi.nlm.nih.gov/geo/) accession GSE233649.