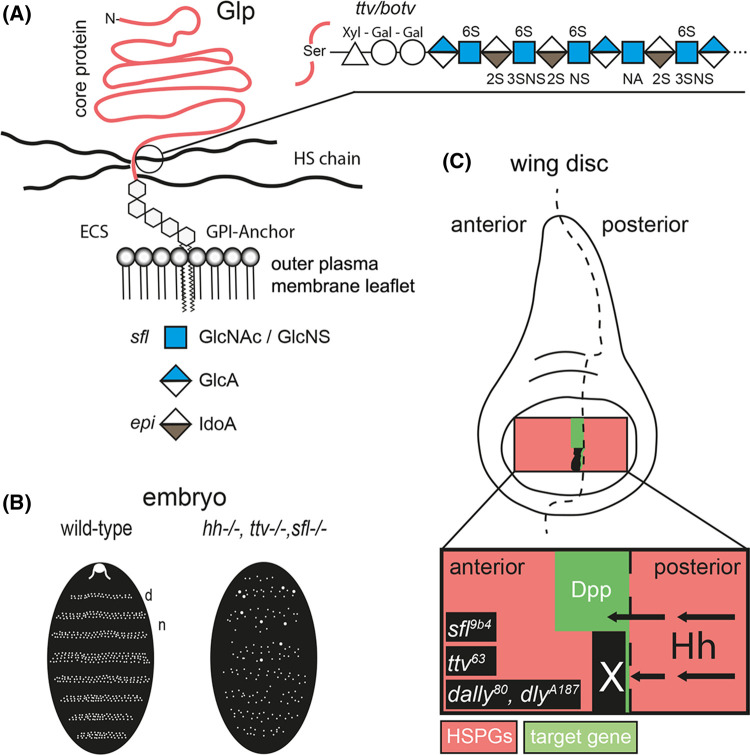
Figure 1. Glp HSPGs are essential for Hh spread.
(A) HS-decorated Glps localize to the outer plasma membrane leaflet and are exposed to extracellular binders. After biosynthesis of the xylose–galactose–galactose–glucuronic acid linker to a serine side chain of the Glp core protein, HS grows by Ttv/Botv-catalyzed copolymerization of GlcAβ1,4 and GlcNAcα1,4 and undergoes modification by Sfl-mediated removal of acetyl groups from subsets of GlcNAc residues and addition of sulphate to the free amino groups. Subsequent activities of O-sulfotransferases and a GlcA C5-epimerase (Epi) complete HS biosynthesis. (B) Scheme of similar patterning defects in Drosophila embryos upon inactivation of hh or HS biosynthetic enzymes. In both cases, the organization of repeating denticle (d) and naked (n) parasegments in mutant embryos which normally decorate Drosophila embryos and first instar larvae is perturbed. (C) Schematic of clonal analysis showing that the absence of Glp HSPGs blocks transport of Hh produced in the posterior compartment of the Drosophila wing disc to the receiving anterior compartment (arrows) [20]. Instead, Hh accumulates in front of cell clones deficient for the gene sulphateless (sfl9b4; this mutant expresses undersulphated HSPGs) and ttv63 (this mutant expresses Glp core proteins without HS chains) in the anterior compartment (marked in black). Of note, Hh fails to cross more than one or two HSPG mutant cells in the anterior compartment (black field with white X), as indicated by the complete absence of dpp (green) target gene expression in the clone as well as in wild-type cells anterior to small clones. HS is therefore required for the spreading of Hh in the anterior (e.g. Hh receiving) compartment. Schemes represent findings as described in [16,20].