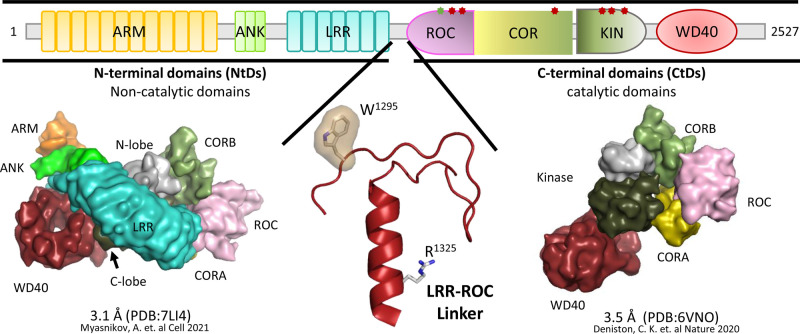
Figure 1. Domain organization of LRRK2 and structures of fl-LRRK2 and LRRK2RCKW.
The domain organization of LRRK2 is summarized at the top, including key Parkinson Disease Mutations. In addition to the seven well-folded domains in full-length inactive LRRK2 there is an additional stable motif that links the LRR domain and the ROC domain. This motif, LRR–ROC Linker (bottom, middle), is disordered in active fl-LRRK2 and missing in LRRK2RCKW. Two key residues in this motif are W1295 described previously (JMB) and R1325 which was recently described as a PD mutation site [3,20]. Here we explore the domain dynamics in two cryo-EM structures. On the bottom left is inactive fl-LRRK2INACT (pdb: 7li4); on the right bottom is LRRK2RCKW (pdb: 6vno). GaMD simulations create a dynamic portrait of each static cryo-EM structure.