Figure 1.
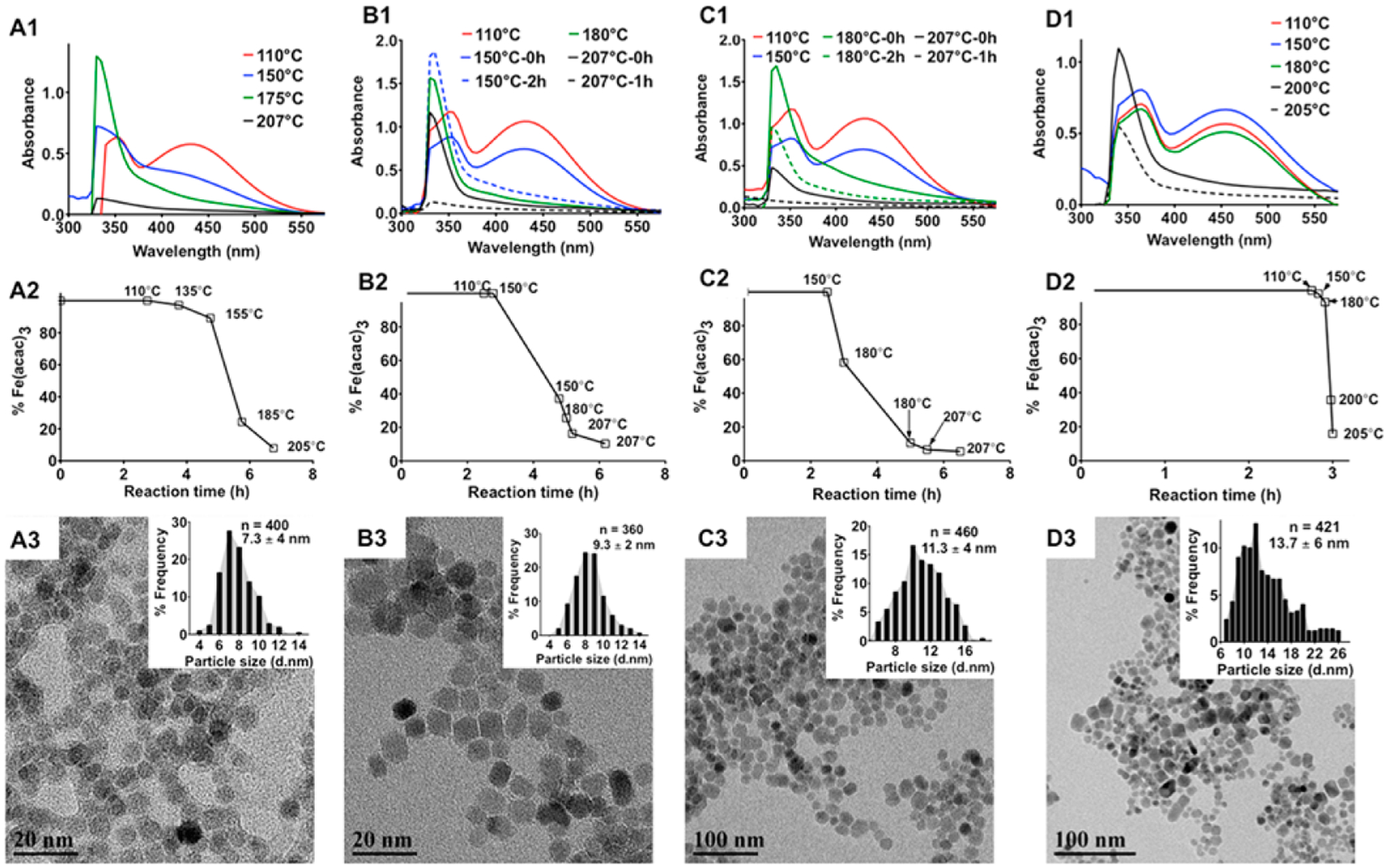
Effect of the heating patterns on the size of MNPs. A1-D1: UV-absorption spectra of the reaction mixtures upon different heating patterns as depicted in Supporting Information Figure S1. Aliquots of the reaction mixture were collected at specific time intervals, precipitated in acetone, and centrifuged to separate any formed MNPs, and the absorbance spectrum of the supernatant was measured to determine the nucleation and growth phases during the synthesis of MNPs. The solid lines correspond to measurement taken at the start of the incubation periods. The dashed lines are spectra taken 2 h after incubation of the system at a given temperature except for D1 where the spectra were taken at the start of reflux. A2-D2: Amount of unreacted Fe(acac)3 in the reaction mixture at the corresponding temperatures during the ramp phase of the heating process as measured by ICP-MS. A3-D3: TEM images of the MNPs synthesized by different heating patterns. Particle size distributions are shown in the respective insets. Diameters of the MNPs were calculated from the TEM images using ImageJ software.
