Abstract
Objectives Sinonasal mucosal melanoma (SNMM) is an extremely rare and challenging sinonasal malignancy with a poor prognosis. Standard treatment involves complete surgical resection, but the role of adjuvant therapy remains unclear. Crucially, our understanding of its clinical presentation, course, and optimal treatment remains limited, and few advancements in improving its management have been made in the recent past.
Methods We conducted an international multicenter retrospective analysis of 505 SNMM cases from 11 institutions across the United States, United Kingdom, Ireland, and continental Europe. Data on clinical presentation, diagnosis, treatment, and clinical outcomes were assessed.
Results One-, three-, and five-year recurrence-free and overall survival were 61.4, 30.6, and 22.0%, and 77.6, 49.2, and 38.3%, respectively. Compared with disease confined to the nasal cavity, sinus involvement confers significantly worse survival; based on this, further stratifying the T3 stage was highly prognostic ( p < 0.001) with implications for a potential modification to the current TNM staging system. There was a statistically significant survival benefit for patients who received adjuvant radiotherapy, compared with those who underwent surgery alone (hazard ratio [HR] = 0.74, 95% confidence interval [CI]: 0.57–0.96, p = 0.021). Immune checkpoint blockade for the management of recurrent or persistent disease, with or without distant metastasis, conferred longer survival (HR = 0.50, 95% CI: 0.25–1.00, p = 0.036).
Conclusions We present findings from the largest cohort of SNMM reported to date. We demonstrate the potential utility of further stratifying the T3 stage by sinus involvement and present promising data on the benefit of immune checkpoint inhibitors for recurrent, persistent, or metastatic disease with implications for future clinical trials in this field.
Keywords: sinonasal mucosal melanoma, TNM, immunotherapy, immune checkpoint blockade, immune checkpoint inhibitors ipilimumab
Introduction
Sinonasal mucosal melanoma (SNMM) is a rare, aggressive, and challenging malignancy comprising 4% of all sinonasal malignancies. Tumors are often detected at a late stage resulting in poor patient prognosis, with 5-year overall survival (OS) below 25%. 1 2 3 The standard of care comprises surgical resection, with comparable outcomes between open and endoscopic approaches in well-selected patients. 4 5 The efficacy of adjuvant radiotherapy and the use of systemic therapy are controversial. 6 7 8 Most patients will experience persistent disease or recurrence, for which treatment options are limited. Distant metastasis is the most common cause of treatment failure, having been reported in 35% of patients. 2
To improve SNMM patient survival outcomes, the use of biochemotherapy and immunotherapy has been the subject of research for the past two decades. Based on the efficacy of biochemotherapy along with interferons and/or interleukins in cutaneous melanoma, it has been widely used as part of adjuvant therapy for the management of SNMM. However, its safety and efficacy are unclear and remain to be elucidated in this disease type. Importantly, due to a lack of large-scale studies, the use of biochemotherapy for SNMM has significantly decreased in recent years. FDA-approved immune checkpoint inhibitors, ipilimumab, pembrolizumab, and nivolumab, have been used for the treatment of SNMM, particularly in the metastatic setting, but no formal trials have been completed to date. Preliminary evidence from a small case study of SNMM has demonstrated the potential efficacy of these drugs, with durable response and acceptable toxicities in two distant metastatic cases. 9 In their analysis of the National Cancer Database, Ganti et al suggested improved survival in patients exhibiting metastatic disease when treated with immunotherapy. 10
Due to the rarity of this malignancy, evidence has been limited to small cohort studies or case series and analyses of existing databases. Here, we present the largest cohort of SNMM reported to date, consisting of data from 11 centers across the United States, continental Europe, United Kingdom, and Ireland. We investigated potential prognostic factors, compared treatment approaches, and provided an up-to-date evaluation of immunotherapy for the management of the recurrent or persistent disease.
Materials and Methods
Patients
Deidentified data on 505 SNMM patients diagnosed between 1999 and 2021 were obtained from four institutions in the United States (The University of Texas MD Anderson Cancer Center, Stanford University School of Medicine, Johns Hopkins University School of Medicine, and the University of Pittsburgh School of Medicine), four institutions in continental Europe (University of Insubria, Italy; ASST Spedali Civili-Università degli Studi di Brescia, Italy; Instituto de Investigacion Sanitaria del Principado de Asturias, Spain, and University Hospital Hradec Kralove, Czech Republic), two institutions in the United Kingdom (University College London/University College London Hospitals and University of Manchester), and one in Ireland (Beaumont Hospital). Inclusion criteria required confirmed histopathological diagnosis of SNMM with histological characterization confirmed by head and neck pathologists experienced in the evaluation of SNMM. Data collected included patient demographics, disease status at presentation, treatment details, and patient outcomes. Institutional review board (IRB) approval was obtained from all institutions with further approval for multicenter data analysis from University College London IRB/Research Ethics Committee (UCL REC no. 9609/002; ML/VJL).
Diagnosis and Treatment of Sinonasal Mucosal Melanoma
The date of diagnosis was defined as the date of tissue extraction for histological determination of the diagnosis. Patients were treated as per their respective institution's standard of care, and all institutions involved are tertiary level centers with longstanding experience in the diagnosis and management of this disease.
Statistical Analysis of Clinical Data
The primary aim of this study was to investigate prognostic factors of SNMM patients in terms of disease-free survival (DFS) and OS, calculated from the date of diagnosis and censored at the date the patient was last known to be alive if no event had occurred. DFS and OS were described using the Kaplan–Meier method and log-rank tests. Univariable and multivariable Cox regression analyses were used to derive hazard ratios (HRs), 95% confidence intervals (CIs), and corresponding p -values, both unadjusted and after accounting for other factors. Associations with the following factors were explored: age, sex, smoking status, alcohol consumption, tumor stage (staging of all the included cases was classified as T3 or greater, reflecting the most recent edition of the American Joint Committee on Cancer staging system), extent of disease at presentation, and treatment approach. Statistical significance was defined as two-sided p -value < 0.05. The data analysis was generated using IBM SPSS Statistics for Windows version 27.0 (IBM Corp., Armonk, NY, United States).
Results
Patient Characteristics
The median age of patients at the time of disease diagnosis was 67.0 years (range = 15–93) and 53.7% were females. In total, 48.5 and 44.2% of patients had a history of tobacco use and alcohol consumption, respectively ( Tables 1 and 2 ).
Table 1. Frequency of clinical characteristics at presentation.
| n | % | ||
|---|---|---|---|
| Gender | Male | 233 | 46.1 |
| Female | 271 | 53.7 | |
| Any tobacco use | No | 124 | 51.5 |
| Yes | 117 | 48.5 | |
| Any cigarette smoking | Never | 121 | 51.1 |
| Former | 86 | 36.3 | |
| Current | 30 | 12.7 | |
| Any alcohol consumption | Never | 130 | 55.8 |
| Former | 22 | 9.4 | |
| Current | 81 | 34.8 | |
| Nasal involvement of original tumor at presentation | No | 61 | 12.9 |
| Yes | 411 | 87.1 | |
| Sinus involvement of original tumor at presentation | No | 293 | 59.6 |
| Yes | 199 | 40.4 | |
| Skull base involvement of original tumor at presentation | No | 194 | 74.9 |
| Yes | 65 | 25.1 | |
| Intracranial involvement of original tumor at presentation | No | 260 | 94.5 |
| Yes | 15 | 5.5 | |
| T-Stage | T3 | 239 | 60.1 |
| T4a | 120 | 30.2 | |
| T4b | 39 | 9.8 | |
| N-Stage | N0 | 447 | 91.2 |
| N1 | 43 | 8.8 | |
| M-Stage | M0 | 329 | 94.3 |
| M1 | 20 | 5.7 | |
Table 2. Prevalence of additional surgical findings.
| n | % | |
|---|---|---|
| Bony invasion ( n = 153) | 51 | 33.3 |
| Lymphovascular invasion ( n = 108) | 8 | 7.4 |
| Cartilage invasion ( n = 143) | 20 | 14.0 |
| Perineural invasion ( n = 115) | 13 | 11.3 |
| Angioinvasion ( n = 107) | 8 | 7.5 |
| Dural invasion ( n = 227) | 7 | 3.1 |
| Brain invasion ( n = 174) | 3 | 1.7 |
| Orbital invasion ( n = 210) | 33 | 15.7 |
Most patients presented with T3 disease (239 out of 398, 60.1%), followed by T4a (120 out of 398, 30.2%) and T4b (39 out of 398, 9.8%). At presentation, nodal disease (43 out of 490, 8.8%) and metastatic disease (20 out of 349, 5.7%) were uncommon. The sinuses and nasal cavity were involved in 40.4% (199 out of 492) and 87.1% (411 out of 472) of tumors, respectively. Skull base involvement was observed in 25.1% (65 out of 259) of patients; however, intracranial involvement was rare (15 out of 275, 5.5%) ( Table 1 and 2 ).
The most common surgical findings were bony invasion (51 out of 153; 33.3%), orbital invasion (33 out of 210, 15.7%), cartilage invasion (20 out of 143; 14.0%), and perineural invasion (13 out of 115; 11.3%).
Patient Outcomes and Prognostic Factors
After a median follow-up of 21.3 months ( N = 467), 1-, 3-, and 5-year OS rates were 77.6% (95% CI: 73.4–81.2%), 49.2% (95% CI: 44.2–54.0%), and 38.3% (95% CI: 33.2–43.4%), respectively ( Fig. 1 ). DFS data were available for 309 patients ( Fig. 2 ), with 1-, 3-, and 5-year DFS rates of 61.4% (95% CI: 55.4–66.8%), 30.6% (95% CI: 25.2–36.1%), and 22.0% (95% CI: 17.0–27.5%). For recurrent or persistent disease, these occurred locally, regionally, and locoregionally in 29.7% (76 out of 256), 5.9% (15 out of 256), and 8.6% (22 out of 256) of patients, respectively. Distant metastasis was observed in 55.9% (143 out of 256) of patients.
Fig. 1.
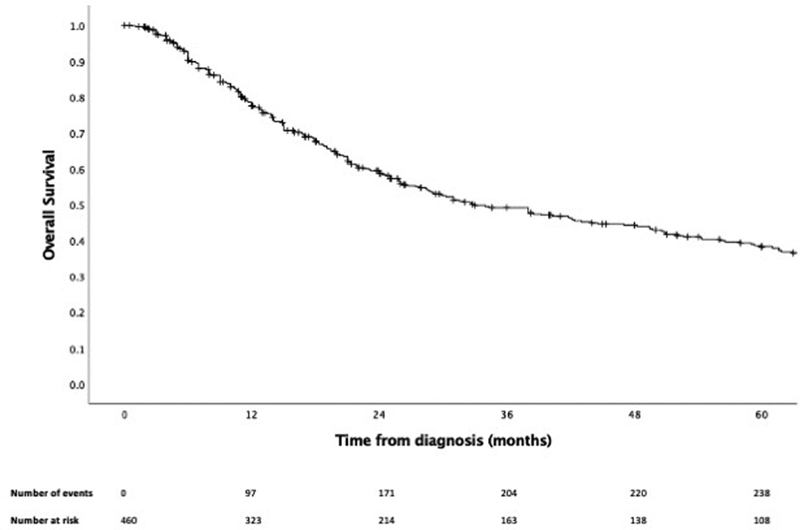
Kaplan–Meier curve of overall survival.
Fig. 2.
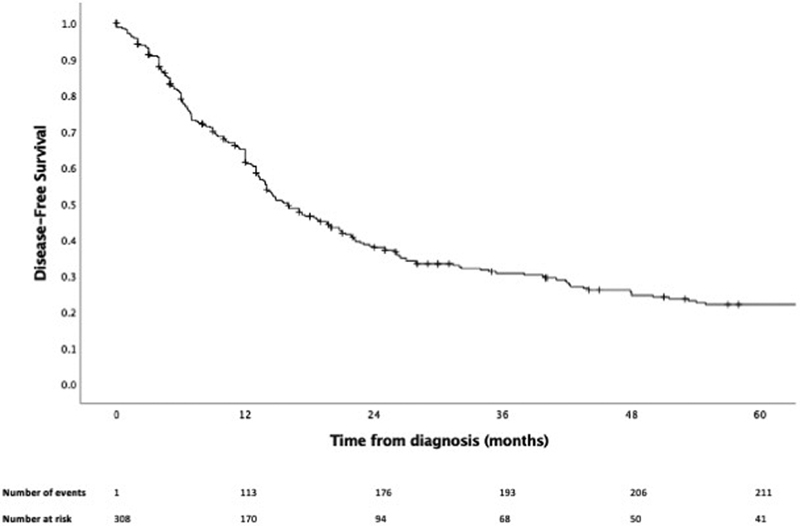
Kaplan–Meier curve of disease-free survival.
Upon univariable survival analysis, there was evidence that higher T-stage (HR T4a versus T3 = 1.32, 95% CI: 0.99–1.75; HR T4b versus T3 = 1.97, 95% CI: 1.27–3.06, p = 0.007), M1-stage disease (HR = 1.88, 95% CI: 1.11–3.19, p = 0.031), sinus involvement (HR = 1.54, 95% CI: 1.21–1.95, p < 0.001), skull base involvement (HR = 1.79, 95% CI: 1.24–2.58, p = 0.003), and intracranial involvement (HR = 3.82, 95% CI: 2.05–7.14, p < 0.001) were associated with worse OS, while a trend toward improved survival was observed for female gender (HR = 0.79, 95% CI: 0.63–1.00, p = 0.052) and nasal involvement (HR = 0.70, 95% CI: 0.49–1.00, p = 0.058). Sinus involvement and intracranial involvement were each independent prognostic factors in multivariable analysis ( Table 3 ).
Table 3. Univariable and multivariable Cox regression overall and recurrence-free survival analyses of clinical and tumor characteristics.
| OS—Univariable | OS—Multivariable | DFS—Univariable | ||||
|---|---|---|---|---|---|---|
| n | HR (95% CI) p -value |
n | HR (95% CI) p -value |
n | HR (95% CI) p -value |
|
| Age | 458 | HR = 1.01 (95% CI: 1.00–1.02) p = 0.113 |
NA | 308 | HR = 1.00 (95% CI: 0.99–1.01) p = 0.919 |
|
| Gender (female vs. male) |
459 | HR = 0.79 (95% CI: 0.63–1.00) p = 0.052* |
231 | HR = 0.89 (95% CI: 0.63–1.25) p = 0.508 |
309 | HR = 0.94 (95% CI: 0.72–1.22) p = 0.626 |
| Tobacco (yes vs. no) |
233 | HR = 1.35 (95% CI: 0.96–1.88) p = 0.082 |
NA | 185 | HR = 0.97 (95% CI: 0.69–1.36) p = 0.851 |
|
| Cigarette smoking | 229 | p = 0.242 | NA | 181 | p = 0.974 | |
| Former vs. never | HR = 1.34 (95% CI: 0.93–1.93) |
HR = 0.1.01 (95% CI: 0.69–1.48) |
||||
| Current vs. never | HR = 1.34 (95% CI: 0.80–2.24) |
HR = 0.95 (95% CI: 0.56–1.62) |
||||
| Alcohol consumption | 225 | p = 0.457 | NA | 178 | p = 0.267 | |
| Former vs. never | HR = 0.77 (95% CI: 0.41–1.45) |
HR = 0.59 (95% CI: 0.28–1.22) |
||||
| Current vs. never | HR = 0.82 (95% CI: 0.57–1.17) |
HR = 1.03 (95% CI: 0.71–1.48) |
||||
| T-Stage | 390 | p = 0.007* | 231 | p = 0.923 | 305 | p = 0.468 |
| T4a vs. T3 | HR = 1.32 (95% CI: 0.99–1.75) |
HR = 0.95 (95% CI: 0.64–1.42) |
HR = 1.12 (95% CI: 0.83–1.50) |
|||
| T4b vs. T3 | HR = 1.97 (95% CI: 1.27–3.06) |
HR = 0.87 (95% CI: 0.42–1.79) |
HR = 1.29 (95% CI: 0.84–1.98) |
|||
| N-Stage (N1 vs. N0) |
452 | HR = 1.33 (95% CI: 0.86–2.05) p = 0.224 |
NA | 306 | HR = 1.51 (95% CI: 0.92–2.49) p = 0.122 |
|
| M-Stage (M1 vs. M0) |
342 | HR = 1.88 (95% CI: 1.11–3.19) p = 0.031* |
231 | HR = 1.87 (95% CI: 0.97–3.63) p = 0.086 |
292 | HR = 1.16 (95% CI: 0.59–2.26) p = 0.674 |
| Nasal involvement (yes vs. no) |
427 | HR = 0.70 (95% CI: 0.49–1.00) p = 0.058 |
NA | 287 | HR = 0.57 (95% CI: 0.38–0.86) p = 0.011* |
|
| Sinus involvement (yes vs. no) |
448 | HR = 1.54 (95% CI: 1.21–1.95) p < 0.001* |
231 | HR = 1.54 (95% CI: 1.07–2.21) p = 0.022* |
300 | HR = 1.15 (95% CI: 0.88–1.50) p = 0.302 |
| Skull base involvement (yes vs. no) |
253 | HR = 1.79 (95% CI: 1.24–2.58) p = 0.003* |
231 | HR = 1.41 (95% CI: 0.89–2.22) p = 0.153 |
209 | HR = 1.02 (95% CI: 0.69–1.51) p = 0.908 |
| Intracranial involvement (yes vs. no) |
268 | HR = 3.82 (95% CI: 2.05–7.14) p < 0.001* |
231 | HR = 3.06 (95% CI: 1.44–6.50) p = 0.007* |
219 | HR = 4.48 (95% CI: 1.94–10.3) p = 0.004* |
| Bony invasion (yes vs. no) |
150 | HR = 1.38 (95% CI: 0.89–2.15) p = 0.160 |
NA | 128 | HR = 1.17 (95% CI: 0;77–1.76) p = 0.469 |
|
| Lymphovascular invasion (yes vs. no) |
106 | HR = 1.37 (95% CI: 0.55–3.46) p = 0.519 |
NA | 90 | HR = 1.57 (95% CI: 0.67–3.63) p = 0.326 |
|
| Cartilage invasion (yes vs. no) |
140 | HR = 1.42 (95% CI: 0.78–2.58) p = 0.265 |
NA | 122 | HR = 1.39 (95% CI: 0.80–2.41) p = 0.261 |
|
| Perineural invasion (yes vs. no) |
113 | HR = 1.17 (95% CI: 0.50–2.70) p = 0.728 |
NA | 93 | HR = 0.91 (95% CI: 0.40–2.11) p = 0.831 |
|
| Angioinvasion (yes vs. no) |
105 | HR = 0.79 (95% CI: 0.29–2.18) p = 0.638 |
NA | 86 | HR = 0.65 (95% CI: 0.24–1.78) p = 0.369 |
|
| Dural invasion (yes vs. no) |
225 | HR = 1.35 (95% CI: 0.59–3.07) p = 0.493 |
NA | 172 | HR = 1.28 (95% CI: 0.56–2.91) p = 0.571 |
|
| Brain invasion (yes vs. no) |
172 | HR = 1.52 (95% CI: 0.48–4.82) p = 0.502 |
NA | 119 | HR = 1.95 (95% CI: 0.48–7.95) p = 0.402 |
|
| Orbital invasion (yes vs. no) |
206 | HR = 1.53 (95% CI: 0.96–2.45) p = 0.088 |
NA | 183 | HR = 1.29 (95% CI: 0.81–2.06) p = 0.294 |
|
Abbreviations: CI, confidence interval; DFS, disease-free survival; HR, hazard ratio; NA, not available; OS, overall survival; RT, radiotherapy.
For DFS, nasal involvement was associated with improved survival (HR = 0.57, 95% CI: 0.38–0.86, p = 0.011), while intracranial involvement was associated with worse survival (HR = 4.48, 95% CI: 1.94–10.3, p = 0.004) ( Table 3 ) upon univariable analysis. No other variables were significantly prognostic of DFS.
On univariable analysis, T-staging was significantly prognostic ( Fig. 3 ), while sinus involvement of the original disease conferred significantly worse outcome ( Fig. 4 ) and, compared with an absence of sinus involvement, was associated with positive surgical margins (37.7 vs. 21.1%, p = 0.008), skull base involvement (34.3 vs. 18.1%, p = 0.004), bony invasion (50.0 vs. 20.2%, p < 0.001), cartilage invasion (25.8 vs. 3.8%, p < 0.001), and orbital invasion (25.4 vs. 4.3%, p < 0.001) ( Supplementary Table S1 , available in the online version). When looking at T-staging, while T4b conferred substantially worse survival, the delineation of the survival curves between T3 and T4a was less clear, prompting us to determine the utility of integrating sinus involvement as part of T-staging. The model of T3 and T4 disease, where T3 was stratified by tumor site being nasal only or involving the sinuses, had a strong prognostic value ( p < 0.001, Fig. 5 ) and demonstrated that there exists a subgroup of patients within T3 disease who have worse survival, at least in part due to sinus involvement, and that this group has a similar outcome to T4a disease. To build on this, a model of T-staging, where T3 with sinus involvement and T4a were combined, was evaluated and found to be significantly prognostic ( p < 0.001, Fig. 6 ).
Fig. 3.

Kaplan–Meier curve of T Staging.
Fig. 4.
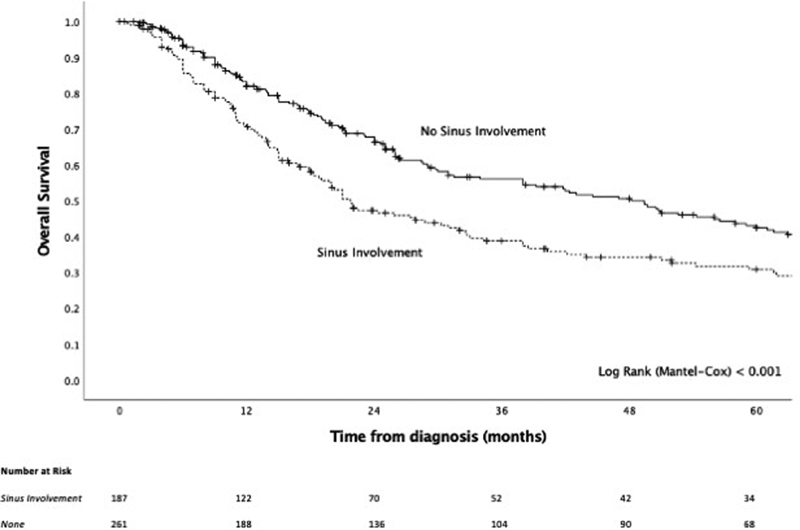
Kaplan–Meier curve of sinus (maxillary, frontal, ethmoid, and/or sphenoid) involvement of the primary tumor.
Fig. 5.
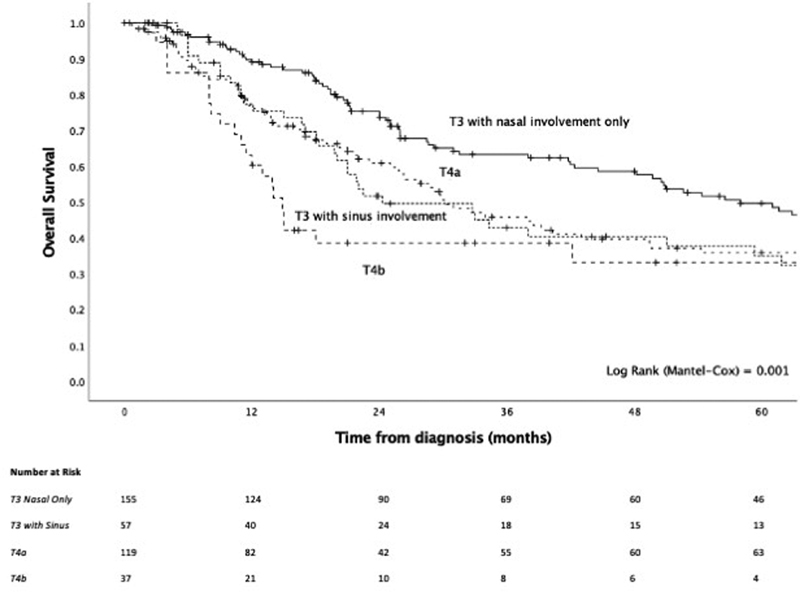
Kaplan–Meier curve of a modified T-staging system, where T3 has been stratified by sinus involvement.
Fig. 6.
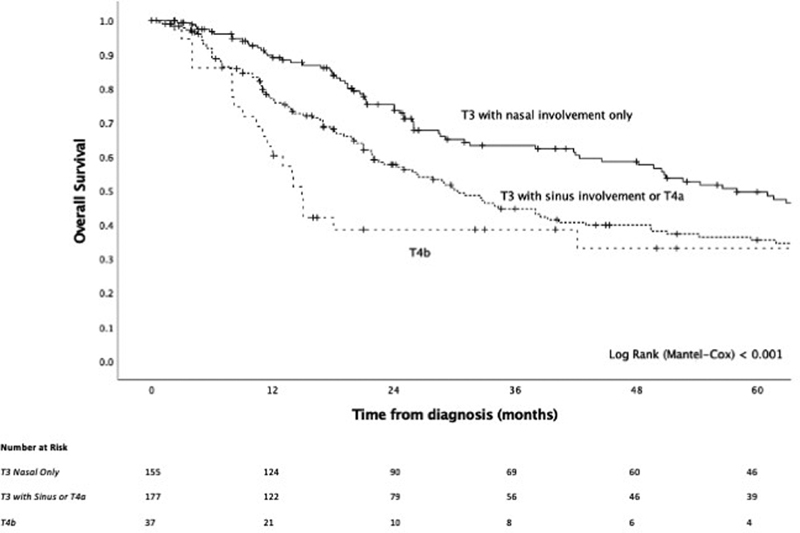
Kaplan–Meier curve of a modified T-staging system, where T3 with sinus involvement has been combined with T4a.
Treatment Approaches and Role of Immunotherapy
Surgery was performed in 89.3% (431 out of 483) of patients, among these 40.7% (197 out of 483) underwent surgery alone, while 44.5% (215 out of 483) received adjuvant radiotherapy as well. Very few patients received adjuvant chemotherapy (54 out of 483, 11.2%) ( Table 4 ). There was evidence that patients who received adjuvant radiotherapy had moderately better OS compared with those who underwent surgery alone (HR = 0.74, 95% CI: 0.57–0.96, p = 0.021, Fig. 7a ), and might have had longer local recurrence-free survival (HR = 0.62, 95% CI: 0.37–1.04, p = 0.066, Fig. 7b ); however, the evidence for the latter is less robust. OS also improved for those who underwent endoscopic resection compared with combined/open surgery (HR = 0.76, 95% CI: 0.58–0.99, p = 0.039, Tables 5 and 6 ), although a selection bias for more limited disease for endoscopic resection was likely. The addition of adjuvant chemotherapy to adjuvant radiotherapy appeared to be detrimental (HR = 1.65, 95% CI: 0.92–2.97, p = 0.114), although the number of patients receiving surgery and adjuvant chemoradiotherapy were small. Moreover, this observation was likely confounded by the severity of the disease which might have informed the treatment approach at the outset ( Table 6 ).
Table 4. Number and frequency of patients who underwent the various treatment approaches.
| n | % | |
|---|---|---|
| None/Biopsy | 9 | 1.9 |
| Excisional biopsy | 1 | 0.2 |
| Surgery only | 197 | 40.8 |
| RT only | 4 | 0.8 |
| Chemotherapy only | 6 | 1.2 |
| Surgery and RT | 192 | 39.8 |
| Surgery and chemotherapy | 19 | 3.9 |
| Chemoradiotherapy | 6 | 1.2 |
| Surgery and chemoradiotherapy | 23 | 4.8 |
| Other | 4 | 0.8 |
| Immunotherapy | 17 | 3.5 |
| Biochemotherapy | 5 | 1.0 |
Abbreviation: RT, radiotherapy.
Fig. 7.
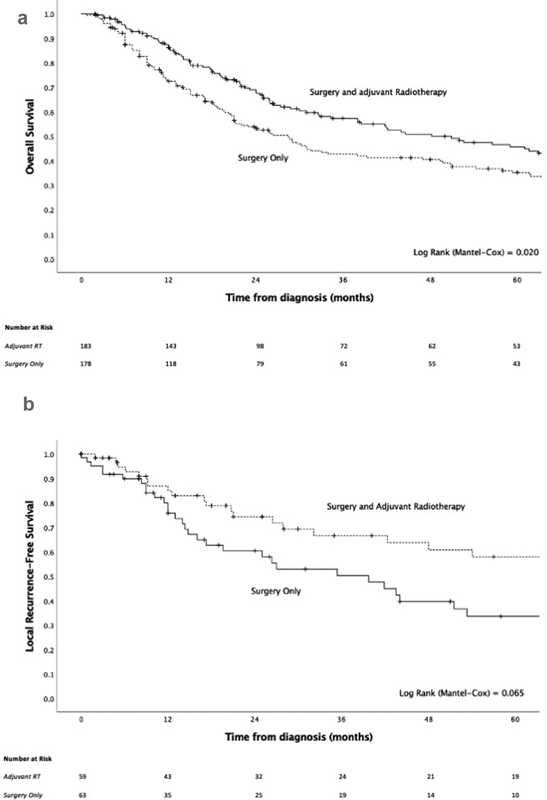
( a ) Kaplan–Meier overall survival curve of surgery only versus surgery and adjuvant radiotherapy for the treatment of disease at presentation. ( b ) Kaplan–Meier local recurrence-free survival curve of surgery only versus surgery and adjuvant radiotherapy for the treatment of disease at presentation.
Table 5. Number and frequency of patients who underwent endoscopic or open/combined surgery.
| n | % | |
|---|---|---|
| Endoscopic resection | 201 | 55.1 |
| Open/Combined | 164 | 44.9 |
Table 6. Univariable Cox regression overall, disease-free, and local recurrence-free survival analyses of treatment approach.
| OS—Univariable | DFS—Univariable | LRFS | ||||
|---|---|---|---|---|---|---|
| n | HR (95% CI) p -value |
n | HR (95% CI) p -value |
n | HR (95% CI) p -value |
|
| Endoscopic versus other surgical approach | 337 | HR = 0.76 (95% CI: 0.58–0.99) p = 0.039 |
217 | HR = 0.81 (95% CI: 0.59–1.10) p = 0.176 |
92 | HR = 0.81 (95% CI: 0.44–1.49) p = 0.495 |
| Surgery and Adj. RT versus surgery alone | 363 | HR = 0.74 (95% CI: 0.57–0.96) p = 0.021 |
254 | HR = 0.83 (95% CI: 0.63–1.10) p = 0.202 |
124 | HR = 0.62 (95% CI: 0.37–1.04) p = 0.066 |
| Surgery and Adj. CRT versus surgery and Adj. RT | 204 | HR = 1.65 (95% CI: 0.92–2.97) p = 0.114 |
147 | HR = 1.49 (95% CI: 0.79–2.78) p = 0.239 |
65 | HR = 1.15 (95% CI: 0.27–4.93) p = 0.852 |
Abbreviations: Adj. adjuvant; CI, confidence interval; CRT, chemoradiotherapy; DFS, disease-free survival; HR, hazard ratio; LRFS, local recurrence-free survival; OS, overall survival; RT, radiotherapy.
For the management of recurrent or persistent disease, with or without distant metastasis ( n = 99), 57.0, 37.4, and 41.4% of patients underwent surgery, radiotherapy, and chemotherapy, respectively, either unimodally or in combination. Interferon and/or interleukin (i.e., biochemotherapy) and immune checkpoint inhibitors (ipilimumab, pembrolizumab, or nivolumab) were administered to 15.2 and 27.3%, respectively, either on their own or as part of multimodal care ( Table 7 ). In exploratory analyses, the addition of immune checkpoint inhibitors at any point in the management of recurrence/persistent disease conferred a significant OS benefit (HR = 0.50, 95% CI: 0.25–1.00, p = 0.036) ( Fig. 8 ). This effect was also seen when considering patients with distant metastatic disease as a single group (HR = 0.25, 95% CI: 0.09–0.74, p = 0.004) ( Fig. 9 ). Conversely, biochemotherapy did not appear to improve survival (HR = 1.76, 95% CI: 0.90–3.43, p = 0.119).
Table 7. Number and frequency of patients who underwent the various treatment approaches for the management of recurrent or persistent disease.
| Count | % | ||
|---|---|---|---|
| Immune checkpoint blockade | No | 72 | 72.7% |
| Yes | 27 | 27.3% | |
| Interferon and/or interleukin | No | 84 | 84.8% |
| Yes | 15 | 15.2% | |
| Chemotherapy | No | 58 | 58.6% |
| Yes | 41 | 41.4% | |
| Surgery | No | 43 | 43.0% |
| Yes | 57 | 57.0% | |
| Radiotherapy | No | 62 | 62.6% |
| Yes | 37 | 37.4% | |
Fig. 8.
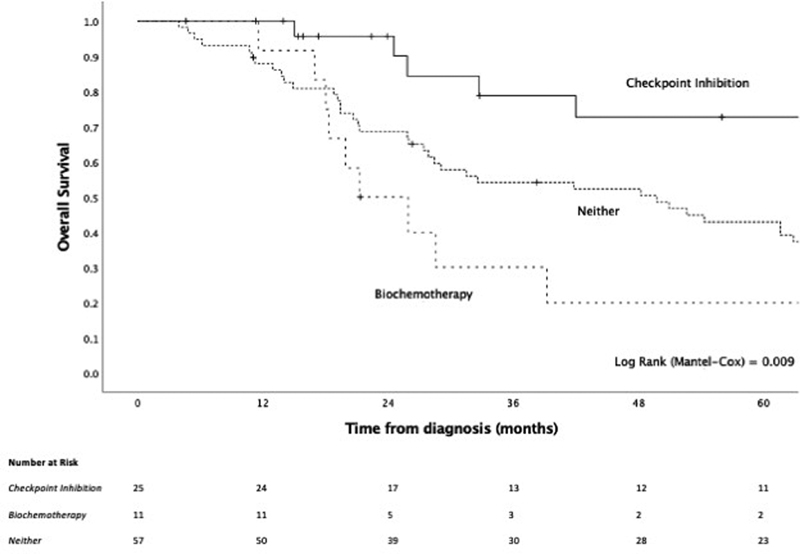
Kaplan–Meier curve of checkpoint inhibition compared with biochemotherapy or neither for the management of recurrent/persistent disease with or without distant metastasis.
Fig. 9.
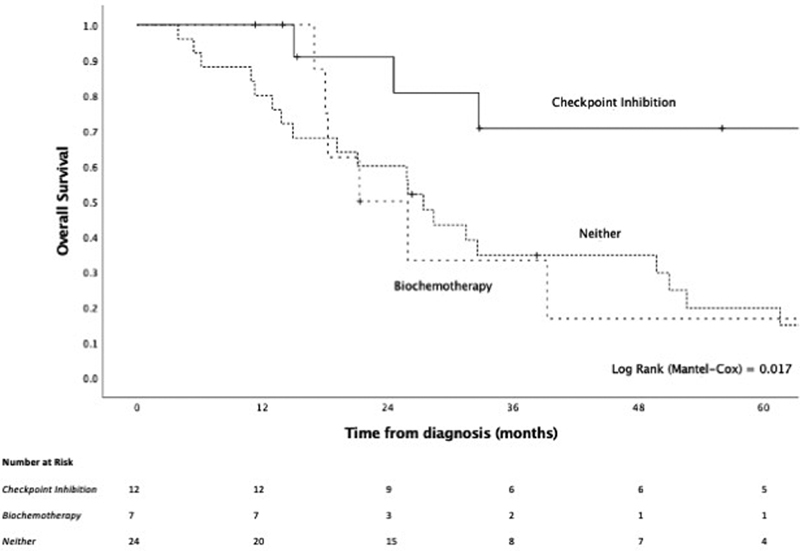
Kaplan–Meier curve of checkpoint inhibition compared with biochemotherapy or neither for the management of recurrent/persistent distantly metastatic disease.
Discussion
This study's findings are based on the largest cohort of SNMM reported to date, comprising an international collaborative effort across 11 tertiary referral centers. Our analysis demonstrates extremely poor outcomes for SNMM, in line with previous literature with half of patients recurring within the first year and 5-year survival of less than 40%.
As previously reported, involvement of the paranasal sinuses confers significantly worse outcomes. 2 11 12 13 14 In the present study, sinus involvement was more common in the maxillary and ethmoids and less frequently observed in the sphenoid or frontal sinuses. Nevertheless, involvement of any of these was associated with a worse outcome. Furthermore, sinus involvement was significantly associated with more invasive disease, confirming previous findings where tumors in the paranasal sinuses had higher rates of local invasion. 2 Some authors postulate that this may be due to delayed diagnosis of disease involving the sinuses and tumors less amenable to surgery due to anatomical constraints. Lastly, while T-staging appears to adequately delineate prognostic groups, in our exploratory analysis, sinus involvement was able to identify a subgroup of T3 cases, which had a worse outcome compared with those with nasal involvement only. Analyzing a series of 18 patients, Houette et al suggest that in addition to standard staging practice, clinical management should consider the tumor site as a significant prognosticator and allocate treatment accordingly. 14 In our cohort, we demonstrate that outcomes of patients with T3 disease with sinus involvement appear to be similar to those with T4a disease. Based on these findings, we propose an adaptation of the currently used tumor, node, and metastasis (TNM) staging system for sinonasal melanoma, i.e., the INSICA (International Network of Sinonasal Cancers; www.insica.org ) modification. If adapted in an updated version of the TNM staging system, this would combine the group of patients with T3 disease with sinus involvement and patients with T4a disease and, in essence, expand the current definition of T4a disease to “T4a: moderately advanced local disease in which tumor involves paranasal sinuses, deep soft tissue, cartilage, bone, or overlying skin” with T3 disease encompassing patients with disease in the nasal cavity only.
Management of SNMM remains challenging with most patients experiencing recurrent, persistent, or distantly metastatic disease. For the treatment of primary disease, current surgical approaches, i.e., open or endoscopic, are comparable in appropriately selected patients. Regarding adjuvant radiotherapy, its use has been controversial, as previously published data suggest that it may only improve local control of disease without impacting OS rates. 15 In the present study, we observed improved OS for those who underwent adjuvant radiotherapy, compared with surgery alone. Furthermore, there was a signal that adjuvant radiotherapy may prolong local recurrence-free survival; however, further studies are warranted to confirm these findings. Moreover, with the prospect of further developments in the field of irradiation, these technological advancements have the potential to be used for this disease, e.g., proton or carbon ion radiation therapy, which has been used in both nonsurgical protocols concurrent to chemotherapy and in the adjuvant setting in head and neck mucosal melanoma. 16 Regarding surgical approach, we did not observe a substantial difference in survival between those who underwent endoscopic resection compared with open/combined approaches, highlighting that endoscopic surgery for well-selected cases is an effective approach, especially when taking into account the potential benefits to the patient's quality of life and morbidity. 4 17 18 Lastly, recent molecular studies showed that a proportion of tumors harbor NRAS, KIT, or BRAF mutations, which are targets for therapies successfully used for other tumors. 19 20 Prospective studies are needed to investigate the efficacy of such agents for the treatment of SNMM.
Half of our cohort experienced distant metastasis, with 44.1% experiencing local/locoregional recurrence. Surgery with or without (chemo)radiotherapy remains the mainstay of treatment for recurrent disease. However, outcomes remain poor. Encouragingly, we observed highly promising survival outcomes with the inclusion of immunotherapy. This was particularly evident with immune checkpoint inhibitors in the multimodal treatment plan for recurrent or persistent local, regional, and distant metastatic disease. We also observed a trend toward an increased use of neoadjuvant immunotherapy, but the numbers in our series limited our analysis and we were unable to draw any meaningful conclusions regarding its efficacy. Further studies are needed to confirm any potential benefit of this approach.
Improved survival of patients with metastatic cutaneous melanoma upon treatment with the anti-CTLA-4 monoclonal antibody, ipilimumab, has been previously demonstrated in a phase 3 randomized controlled trial comparing its use with or without additional glycoprotein 100 peptide vaccine. 21 The safety and efficacy of the anti-PD-1 immune checkpoint inhibitor, nivolumab, have also been demonstrated in mucosal melanoma, with superior outcomes for those who receive combination therapy of ipilimumab and nivolumab. 22 For advanced melanoma and ipilimumab-refractory melanoma, pembrolizumab (anti-PD-1) has also shown to confer antitumor activity. 23 24 In a randomized, controlled, phase 3 study comparing pembrolizumab to ipilimumab in patients with advanced cutaneous melanoma, prolonged progression-free and OS were observed in those who received pembrolizumab. 25 Building on these, double immune checkpoint blockade, comprising a combination of anti-PD-1 and anti-CTLA-4 therapies, has been proposed in recent studies, particularly for the treatment of unresectable melanoma or for patients resistant to a single immunotherapy protocol. 26 27 Based on this and the superior survival observed in those who underwent immune checkpoint blockade for the management of recurrence, persistence, or distant metastasis from cutaneous melanoma, it becomes clear that further prospective studies are warranted. These future studies will confirm the safety and efficacy of these approaches for the management of SNMM, both in the primary and recurrent settings. Intriguingly, there is evidence to suggest that immune checkpoint inhibitors may have a radio-sensitizing effect, and therefore, a combination of adjuvant immunotherapy and radiotherapy may prove to be advantageous and is the subject of an ongoing clinical trial (NCT04017897). 28
Lastly, we observed a substantial improvement with immune checkpoint inhibitors over biochemotherapy alone, which itself does not appear to greatly impact survival. Indeed, while biochemotherapy has been widely used in the past, it has been removed from standard practice at several institutions due to a lack of evidence for its efficacy, as well as a high risk of associated toxicities, in line with the findings in this study.
We acknowledge that our study is limited by its retrospective design; hence, statistical analyses are limited to those of an exploratory nature and results should be considered in this context. Furthermore, inherent to this being a large-scale multicenter cohort study, heterogeneity in the data collected as well as missing data were unavoidable, even though an incredible effort was made to mitigate these.
In summary, this is the largest dataset reported to date on SNMM and offers a much-needed update to our current understanding of this extremely challenging malignancy. We confirm previous findings that the tumor site is significantly prognostic with worse outcomes observed for those with sinus involvement of any kind. We propose a refined staging system that takes this into account. While we could not draw any confirmatory conclusions regarding the role of immunotherapy in the adjuvant setting for primary disease, the beneficial use of immune checkpoint inhibitors for recurrent, persistent, or distantly metastatic disease may be substantial. This is of particular importance as most patients will suffer recurrence or distant metastasis, for which treatment options have traditionally been very limited. In line with our findings, further trials on immune checkpoint inhibitors are warranted in both the neoadjuvant and adjuvant treatment setting for SNMM.
Acknowledgments
We would like to acknowledge the invaluable support from Prof. Tariq Enver and the UCL Cancer Institute. We would also like to thank Josep Linares, Dr. Naomi Guppy, and David Allan from HSL Labs/UCL Advanced Diagnostics.
Funding Statement
Funding This work was supported by the Rhinology and Laryngology Research Fund, Royal College of Surgeons and the UCL/UCLH Biomedical Research Centre (BRC). Additional support was provided by the National Institutes of Health/National Institute on Deafness and Other Communication Disorders.
Conflict of Interest NL receives research funding from Merck Inc., not related to this manuscript and was a consultant for CoolTech Inc. and holds stock in Navigen Pharmaceuticals, both of which are unrelated to this manuscript. All other authors declare no potential relevant conflicts of interest.
Authors' Contributions
M.L., Y.T., M.T.-Z., M.F., J. L., N.C., P.H.H., M.H., P.B., P.C., D.M.B., V.J.L., and E.Y.H. were responsible for conception and design.
M.L., Y.T., M.T.-Z., M.F., J.L., N.C., P.H.H., M.H., P.B., P. C., D.M.B., V.J.L., and E.Y.H. were responsible for the development of methodology.
M.L., Y.T., M.T.-Z., M.F., J.L., D. M., V.R., W.V., D.L., R.S., K.W.P., V.H.S., A.F., C.F., F.S., S.B., T.F., F.M.V., P. O'F., P. S., S.W., S.A.H., S.U., J.H., R.D., C.T.F., P.R., S.G., J.J., P.J. A., M.D., D.T., A.T., T.Z., G.R., C. S., J.E.J., J.L., E.W.W., C.S., P.D.L., R. W., J.P. O'N., A.S., R.P.K., T.Z., M. R. Jr., G.G., N.L., Q.T. L., R.B.W., Z.M.P., J. N., P.H.H., M.H., J.L., F.F., P.N., P. B., P.C., A.J., D.C., M.D.F., D.M.B., V.J. L., and E.Y.H. were responsible for acquisition of data.
M.L., Y.T., M.T.-Z., M.F., J.L., N.C., P.H.H., M.H., P.B., P.C., D.M.B., V.J.L., and E.Y.H. were responsible for analysis and interpretation of data.
M.L., Y.T., M.T.-Z., M.F., J.L., N.C., D.M., D.T., A.E.T., J.E.J., J.L., C.S., R.W., P.H.H., A.S., M.H., P.B., P.C., D.M.B., V.J. L., and E.Y.H. were Writing, review, and/or revision of the manuscript.
M.L., V.J.L., and E.Y.H. were responsible for study supervision.
These authors contributed equally to this article.
Supplementary Material
References
- 1.Lund V J, Chisholm E J, Howard D J, Wei W I. Sinonasal malignant melanoma: an analysis of 115 cases assessing outcomes of surgery, postoperative radiotherapy and endoscopic resection. Rhinology. 2012;50(02):203–210. doi: 10.4193/Rhino.11.267. [DOI] [PubMed] [Google Scholar]
- 2.Amit M, Tam S, Abdelmeguid A S. Patterns of treatment failure in patients with sinonasal mucosal melanoma. Ann Surg Oncol. 2018;25(06):1723–1729. doi: 10.1245/s10434-018-6465-y. [DOI] [PubMed] [Google Scholar]
- 3.Lund V J. Sinonasal malignant melanoma. Adv Otorhinolaryngol. 2020;84:185–196. doi: 10.1159/000457937. [DOI] [PubMed] [Google Scholar]
- 4.Miglani A, Patel S H, Kosiorek H E, Hinni M L, Hayden R E, Lal D. Endoscopic resection of sinonasal mucosal melanoma has comparable outcomes to open approaches. Am J Rhinol Allergy. 2017;31(03):200–204. doi: 10.2500/ajra.2017.31.4435. [DOI] [PubMed] [Google Scholar]
- 5.Hur K, Zhang P, Yu A, Kim-Orden N, Kysh L, Wrobel B. Open versus endoscopic approach for sinonasal melanoma: a systematic review and meta-analysis. Am J Rhinol Allergy. 2019;33(02):162–169. doi: 10.1177/1945892418822637. [DOI] [PubMed] [Google Scholar]
- 6.Meleti M, Leemans C R, de Bree R, Vescovi P, Sesenna E, van der Waal I. Head and neck mucosal melanoma: experience with 42 patients, with emphasis on the role of postoperative radiotherapy. Head Neck. 2008;30(12):1543–1551. doi: 10.1002/hed.20901. [DOI] [PubMed] [Google Scholar]
- 7.Ajmani G S, Liederbach E, Kyrillos A, Wang C H, Pinto J M, Bhayani M K. Adjuvant radiation and survival following surgical resection of sinonasal melanoma. Am J Otolaryngol. 2017;38(06):663–667. doi: 10.1016/j.amjoto.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Gore M R, Zanation A M. Survival in sinonasal melanoma: a meta-analysis. J Neurol Surg B Skull Base. 2012;73(03):157–162. doi: 10.1055/s-0032-1301400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manton T, Tillman B, McHugh J, Bellile E, McLean S, McKean E. Sinonasal melanoma: a single institutional analysis and future directions. J Neurol Surg B Skull Base. 2019;80(05):484–492. doi: 10.1055/s-0038-1676355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ganti A, Raman A, Shay A. Treatment modalities in sinonasal mucosal melanoma: a national cancer database analysis. Laryngoscope. 2020;130(02):275–282. doi: 10.1002/lary.27995. [DOI] [PubMed] [Google Scholar]
- 11.Dauer E H, Lewis J E, Rohlinger A L, Weaver A L, Olsen K D. Sinonasal melanoma: a clinicopathologic review of 61 cases. Otolaryngol Head Neck Surg. 2008;138(03):347–352. doi: 10.1016/j.otohns.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 12.Khan M N, Kanumuri V V, Raikundalia M D. Sinonasal melanoma: survival and prognostic implications based on site of involvement. Int Forum Allergy Rhinol. 2014;4(02):151–155. doi: 10.1002/alr.21243. [DOI] [PubMed] [Google Scholar]
- 13.Roth T N, Gengler C, Huber G F, Holzmann D. Outcome of sinonasal melanoma: clinical experience and review of the literature. Head Neck. 2010;32(10):1385–1392. doi: 10.1002/hed.21340. [DOI] [PubMed] [Google Scholar]
- 14.Houette A, Gilain L, Mulliez A, Mom T, Saroul N. Prognostic value of two tumour staging classifications in patients with sinonasal mucosal melanoma. Eur Ann Otorhinolaryngol Head Neck Dis. 2016;133(05):313–317. doi: 10.1016/j.anorl.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 15.Umeda Y, Yoshikawa S, Kiniwa Y. Real-world efficacy of anti-PD-1 antibody or combined anti-PD-1 plus anti-CTLA-4 antibodies, with or without radiotherapy, in advanced mucosal melanoma patients: a retrospective, multicenter study. Eur J Cancer. 2021;157:361–372. doi: 10.1016/j.ejca.2021.08.034. [DOI] [PubMed] [Google Scholar]
- 16.Working Group on Head and Neck Tumors . Takayasu Y, Kubo N, Shino M. Carbon-ion radiotherapy combined with chemotherapy for head and neck mucosal melanoma: prospective observational study. Cancer Med. 2019;8(17):7227–7235. doi: 10.1002/cam4.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swegal W, Koyfman S, Scharpf J. Endoscopic and open surgical approaches to locally advanced sinonasal melanoma: comparing the therapeutic benefits. JAMA Otolaryngol Head Neck Surg. 2014;140(09):840–845. doi: 10.1001/jamaoto.2014.1321. [DOI] [PubMed] [Google Scholar]
- 18.Castelnuovo P, Lepera D, Turri-Zanoni M. Quality of life following endoscopic endonasal resection of anterior skull base cancers. J Neurosurg. 2013;119(06):1401–1409. doi: 10.3171/2013.8.JNS13296. [DOI] [PubMed] [Google Scholar]
- 19.Turri-Zanoni M, Medicina D, Lombardi D. Sinonasal mucosal melanoma: Molecular profile and therapeutic implications from a series of 32 cases. Head Neck. 2013;35(08):1066–1077. doi: 10.1002/hed.23079. [DOI] [PubMed] [Google Scholar]
- 20.Amit M, Tam S, Abdelmeguid A S. Mutation status among patients with sinonasal mucosal melanoma and its impact on survival. Br J Cancer. 2017;116(12):1564–1571. doi: 10.1038/bjc.2017.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hodi F S, O'Day S J, McDermott D F. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(08):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D'Angelo S P, Larkin J, Sosman J A. Efficacy and safety of nivolumab alone or in combination with ipilimumab in patients with mucosal melanoma: a pooled analysis. J Clin Oncol. 2017;35(02):226–235. doi: 10.1200/JCO.2016.67.9258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ribas A, Hamid O, Daud A. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA. 2016;315(15):1600–1609. doi: 10.1001/jama.2016.4059. [DOI] [PubMed] [Google Scholar]
- 24.Ribas A, Puzanov I, Dummer R. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16(08):908–918. doi: 10.1016/S1470-2045(15)00083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.KEYNOTE-006 investigators . Robert C, Schachter J, Long G V. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 26.Li J, Kan H, Zhao L, Sun Z, Bai C. Immune checkpoint inhibitors in advanced or metastatic mucosal melanoma: a systematic review. Ther Adv Med Oncol. 2020;12:1.758835920922028E15. doi: 10.1177/1758835920922028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rose A AN, Armstrong S M, Hogg D. Biologic subtypes of melanoma predict survival benefit of combination anti-PD1+anti-CTLA4 immune checkpoint inhibitors versus anti-PD1 monotherapy. J Immunother Cancer. 2021;9(01):e001642. doi: 10.1136/jitc-2020-001642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim H J, Chang J S, Roh M R. Effect of radiotherapy combined with pembrolizumab on local tumor control in mucosal melanoma patients. Front Oncol. 2019;9:835. doi: 10.3389/fonc.2019.00835. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


