Abstract
Background
This study aimed to devise a simple assessment system for bone metastases (BMs) from lung cancer (LC).
Methods
A total of 368 LC patients with BMs who underwent radiotherapy (RT) were retrospectively reviewed. Prognostic factors were evaluated using multivariate analysis, and a scoring system based on regression coefficients was devised.
Results
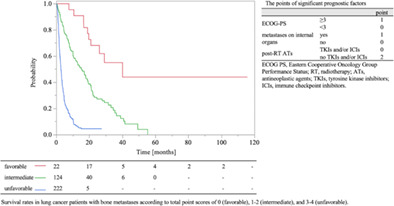
The median follow‐up time for survival was 4.3 months, and the 0.5‐year overall survival (OS) rate was 44.7%. In the multivariate analysis, the significant prognostic factors were performance status (PS), metastases to internal organs, and post‐RT molecular‐targeting therapies (MTs) (tyrosine kinase inhibitors, and/or immune checkpoint inhibitors). A scoring system aggregating points assigned to each risk factor was created (2 points; non‐administration of post‐RT MTs, 1 point; PS ≥3 and metastases to internal organs). The median OSs were 25.0 months, 12.8 months, and 2.5 months in patients with a total score of 0 (n = 22), 1–2 (n = 124), and 3–4 (n = 221), respectively (p < 0.01).
Conclusion
This easy‐to‐use scoring system is useful for selecting patients who received comparatively high‐dose fractionated RT for BMs from LC. Updates are required to follow the progress of systemic therapy.
Keywords: bone metastasis, individualized therapy, lung cancer, palliative radiotherapy, prognostic factor
Our study proposed an easy‐to‐use scoring system for selecting the lung cancer patients with bone metastases for optimal radiation dose.
INTRODUCTION
Bone is the third most frequent site of metastasis, following the lungs and liver. 1 Bone metastases (BMs) occur in ~30% of metastatic lung cancer (LC). 2 Patients with BMs are often treated using a multidisciplinary approach and many receive palliative radiotherapy (RT) for BMs. Regarding BMs from various cancers, most patients who require palliative RT for BMs cannot expect long‐term survival. Svensson et al. 3 reported that 1‐year survival after the diagnosis of BMs was lowest in LC patients (10%). Therefore, single‐fraction RT is often performed for BMs.
Recently, remarkable progress in systemic therapy, mainly using tyrosine kinase inhibitors (TKI) or immune checkpoint inhibitors (ICI), has improved the prognosis of patients with advanced LC. 4 , 5 , 6 , 7 , 8 Although single‐fraction RT is as effective as fractionated RT for pain relief in BMs, 9 previous studies have shown that local control for BMs is insufficient with single‐fraction RT, especially in long‐term survivors. 10 , 11 , 12 , 13 The prediction of life expectancy seems to have become more important for selecting palliative RT doses for BMs than ever in the individualized medicine era.
Many studies devising a prognostic scoring system for patients with BMs have included various primary cancers and do not consider the types of systemic therapy as subsequent treatment. 14 , 15 , 16 In contrast, Katagiri et al. 17 classified LC into moderate growth (LC treated with TKIs) or rapid growth (LC without TKIs) cancer types in their prognostic scoring system for patients with BMs from various cancers. However, they did not evaluate ICI administration. Furthermore, although their classification was very precise because the same scoring system was used to assess the prognosis of patients with BMs from various cancers, a simple predictive scoring system is desirable because of the many factors to be considered in their scoring system. Therefore, to select the optimal RT dose for BMs from LC, we assessed prognostic factors in LC patients with BMs and devised a prognostic scoring system using factors that are easily evaluable in daily clinical practice without the need for sophisticated or costly methods.
METHODS
Patients
Between January 2011 and December 2021, 368 consecutive LC patients were treated with initial RT for BMs at our institution. These LC patients with BMs were referred from an attending physician to a radiation oncologist for palliative RT for the following reasons: (1) pain relief, (2) metastatic spinal cord compression with or without pain and/or neurological symptoms. The total number of LC patients with BMs, including patients not requiring palliative RT, could not be identified. This observational, retrospective cohort study was approved by the Institutional Ethics Review Board of our institution (RIN2021‐70).
BMs were detected using computed tomography (CT), bone scintigraphy, or 18F fluorodeoxyglucose positron‐emission tomography and CT (FDG‐PET/CT) scans. Performance status (PS) was evaluated using the Eastern Cooperative Oncology Group scale.
Radiotherapy
Patients received three‐dimensional conformal RT delivered using 4–10 MV photons with a linear accelerator (Clinac 21EX or TrueBeam, Varian Medical Systems). Patients treated with precise irradiation methods, such as intensity‐modulated radiation therapy or stereotactic body radiation therapy (SBRT), were not included in this study.
Most patients received 30 Gy in 10 fractions (n = 233, 63.3%). The other fraction schedules were as follows: 1 × 8 Gy (n = 13), 5–7 × 4 Gy (n = 56), 10–16 × 2.5 Gy (n = 36), 12–16 × 3 Gy (n = 15), 25 × 2 Gy (n = 1), 8 × 3.6 Gy (n = 6), 5 × 4 Gy + 3 × 3 Gy (n = 7), and 5 × 4 Gy + 2 × 3.6 Gy (n = 1).
Statistical analyses
Survival rate, defined as the time from the beginning of RT until death, was calculated using the Kaplan–Meier method. The Cox proportional hazard model to determine hazard ratios (HRs), including 95% confidence intervals (CIs) and p‐values, was used for the univariate and multivariate analyses. Factors, including age, sex, PS, histology, smoking history, metastases appearing at recurrence or de novo metastases, metastases to internal organs, multiple BMs, bone metastatic sites, antineoplastic agent therapy (AT) before or after RT (pre‐RT or post‐RT ATs), and laboratory data before RT (pre‐RT laboratory data), were analyzed using univariate analysis. Post‐RT ATs were defined as antineoplastic agents administered immediately after RT as this information is available at the start of RT in clinical practice. Factors with p‐values <0.05 on univariate analysis were subjected to multivariate analysis and a scoring system based on regression coefficients in the multivariate analysis was devised. Internal and external validity of this scoring system was assessed using a χ2 goodness of fit test as a measure of calibration 18 and the area under the receiver operating characteristic curve as a measure of discrimination. 19 The area under the curve (AUC) was used as a measure of the discriminative value of the scoring system. 20 Statistical analyses were performed using the JMP software (JMP version 14.3.0; SAS Institute).
RESULTS
Patient characteristics are listed in Table 1. Ninety (24.5%), 43 (11.7%), and 235 (63.9%) patients had single, 2–3, and >3 BMs, respectively. One hundred and eighty‐nine (51.4%) patients had undergone pre‐RT ATs, including chemotherapy, TKIs, or ICIs, and 194 (52.7%) patients had undergone post‐RT ATs. ATs were divided into two groups (molecular‐targeting therapies [MTs], including TKIs or ICIs, vs. others) according to the 0.5‐year overall survival (OS) of each initial treatment of post‐RT ATs (TKIs, 87.1%; ICIs, 80.1%; chemotherapy, 48%; no ATs, 14.1%).
TABLE 1.
Patients characteristics
| Characteristic | No. of patients | % | |
|---|---|---|---|
| Age, years | <70 | 188 | 51.1 |
| ≥70 | 180 | 48.9 | |
| Sex | Male | 242 | 65.8 |
| Female | 126 | 34.2 | |
| ECOG‐PS | 0–1 | 158 | 42.9 |
| 2 | 103 | 28.0 | |
| 3–4 | 107 | 29.1 | |
| Histology | Adenocarcinoma | 247 | 67.1 |
| Squamous cell carcinoma | 53 | 14.4 | |
| Small cell carcinoma | 39 | 10.6 | |
| Other | 29 | 7.9 | |
| Smoking history | Yes | 228 | 72.2 |
| No | 88 | 27.8 | |
| Timing of RT | De novo | 136 | 37.0 |
| Relapse or appearance | 232 | 63.0 | |
| Metastases on internal organs | Yes | 292 | 79.3 |
| Single | 130 | 44.0 | |
| Multiple | 162 | 35.3 | |
| No | 76 | 20.7 | |
| No. of bone metastatic lesions | 1 | 90 | 24.5 |
| 2–3 | 43 | 11.7 | |
| ≥4 | 235 | 63.9 | |
| Bone metastatic sites | Only vertebral | 85 | 23.1 |
| Only non‐vertebral | 62 | 16.8 | |
| Others | 221 | 60.0 | |
| Neurological symptom | Yes | 74 | 20.1 |
| No | 294 | 79.9 | |
| Pathological fracture | Yes | 64 | 17.4 |
| No | 304 | 82.6 | |
| RT course | Shorter course RT | 83 | 22.6 |
| Longer course RT | 285 | 77.4 | |
| Pre‐RT ATs | Yes | 189 | 51.4 |
| TKIs | 49 | 13.3 | |
| ICIs | 32 | 8.7 | |
| TKIs + ICIs | 2 | 0.5 | |
| Other ATs | 106 | 28.8 | |
| No | 179 | 48.6 | |
| Post‐RT ATs | Yes | 194 | 52.7 |
| TKIs | 87 | 23.6 | |
| ICIs | 31 | 8.4 | |
| Other ATs | 76 | 20.7 | |
| No | 174 | 47.3 | |
| Pre‐RT laboratory data, median [range] | CRP (mg/dL) | 1.58 [0.01–110.7] | |
| LDH (U/L) | 257 [119–10 573] | ||
| Albumin (g/dL) | 3.6 [1.7–4.9] | ||
| Platelet (×104/μL) | 23.2 [2.7–147.6] | ||
| Ca (mg/dL) | 9.1 [5.8–22.5] | ||
| T‐Bil (mg/dL) | 0.46 [0.15–28.0] | ||
Abbreviations: ATs, antineoplastic agents; Ca, calcium; CRP, C‐reactive protein; de novo, bone metastases eligible for palliative RT at presentation; ECOG PS, Eastern Cooperative Oncology Group Performance Status; ICIs, immune checkpoint inhibitors; LDH, lactate dehydrogenase; relapse or appearance, bone metastases not eligible for palliative RT at presentation, or appeared after definitive treatment; RT, radiotherapy; T‐Bil, total bilirubin; TKIs, tyrosine kinase inhibitors.
Regarding pre‐RT laboratory data, remarkably few patients showed critical abnormal laboratory data (platelets, 0; serum calcium, 14; total albumin, 5). Therefore, abnormal (C‐reactive protein [CRP] ≥0.4 mg/dL, lactate dehydrogenase [LDH], ≥250 IU/L, or serum albumin <3.7 g/dL) and critical abnormal (platelet <100 000/L, serum calcium ≥10.3 mg/dL, or total bilirubin ≥1.4 mg/dL) laboratory data were included within the same group as abnormal laboratory data.
A total of 269 (73.1%) patients underwent contrast‐enhanced T1‐weighted magnetic resonance imaging (CE‐MRI), 96 (26.1%) patients underwent CT or FDG‐PET/CT, and three (0.8%) patients did not undergo any evaluation for brain metastases. Brain metastases were detected in 139 (37.8%) patients.
Among the 368 patients, 299 (81.3%) died, and 69 (18.7%) were alive at the latest follow‐up. The median follow‐up duration of OS was 4.3 months (range, 0.1–115.6 months), and the 0.5‐ and 1‐year OS rates were 44.7% and 29.4%, respectively (Figure 1). In addition, the median follow‐up duration for survival of living and dead patients at final follow‐up was 17.3 months (range, 0.8–115.6 months) and 3.6 months (range, 0.1–55.2 months), respectively.
FIGURE 1.
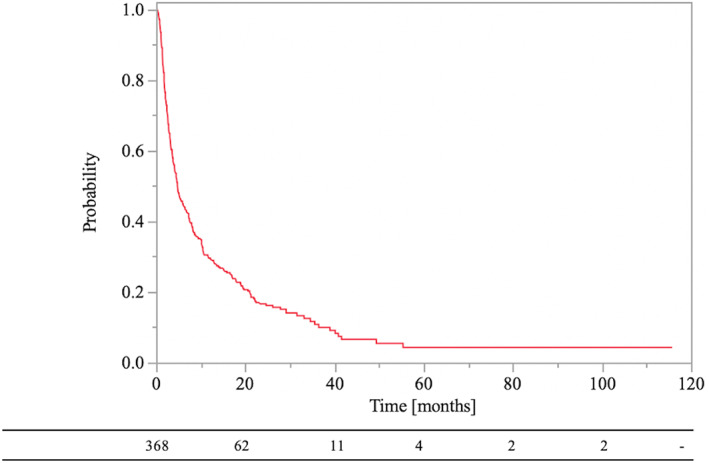
Kaplan–Meier curves of overall survival rate in lung cancer patients with bone metastases.
Prognostic factors for patients with BMs from LC
In the univariate analysis, PS (<3 vs. ≥3; HR, 1.50; 95% CI, 1.19–1.89; p < 0.01), smoking history (no vs. yes; HR, 1.49; 95% CI, 1.12–1.98; p = 0.01), metastases to internal organs (no vs. yes; HR, 1.87; 95% CI, 1.39–2.52: p < 0.01), number of bone metastatic lesions (single vs. multiple; HR, 1.55; 95% CI, 1.19–2.03; p < 0.01), post‐RT ATs (MTs vs. others; HR, 4.38; 95% CI, 3.32–5.79; p < 0.01), and pre‐RT laboratory data (normal vs. abnormal; HR, 2.01; 95% CI, 1.27–3.17; p < 0.01) were significantly associated with OS (Table 2).
TABLE 2.
Survival rates after EBRT and results of univariate and multivariate analyses
| 0.5‐year (%) | 1‐year (%) | Univariate analysis | Multivariate analysis | Regression coefficient | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |||||
| Age | <70 years vs. ≥70 years | 51.3 vs. 37.5 | 34.0 vs. 24.3 | 1.23 (0.98–1.55) | 0.07 | – | – | – |
| Sex | Female vs. male | 49.7 vs. 42.1 | 38.5 vs. 24.6 | 1.25 (0.98–1.59) | 0.07 | – | – | – |
| ECOG‐PS | 0–2 vs. 3–4 | 52.6 vs. 32.9 | 35.7 vs. 20.8 | 1.50 (1.19–1.89) | <0.01 | 1.59 (1.22–2.07) | <0.01 | 0.24 |
| Histology | NSCLC vs. SCLC | 45.4 vs. 38.4 | 30.4 vs. 20.7 | 1.21 (0.84–1.75) | 0.30 | – | – | – |
| Smoking history | No vs. yes | 58.7 vs. 43.1 | 46.2 vs. 25.9 | 1.49 (1.12–1.98) | 0.01 | 1.03 (0.76–1.39) | 0.86 | 0.02 |
| Timing of RT | De novo vs. relapse or appearance | 47.5 vs. 43.2 | 34.2 vs. 27.0 | 1.12 (0.88–1.42) | 0.37 | – | – | – |
| Metastases on internal organs | No vs. yes | 65.6 vs. 39.3 | 48.7 vs. 24.4 | 1.87 (1.39–2.52) | <0.01 | 1.86 (1.33–2.60) | <0.01 | 0.31 |
| Number of bone metastatic lesions | 1 vs. ≥2 | 63.7 vs. 38.4 | 40.5 vs. 25.7 | 1.55 (1.19–2.03) | <0.01 | 1.28 (0.97–1.69) | 0.08 | 0.13 |
| Bone metastatic sites | Only spine vs. others | 51.6 vs. 42.6 | 36.3 vs. 27.7 | 1.21 (0.92–1.59) | 0.16 | – | – | – |
| Neurological symptom | No vs. yes | 47.1 vs. 35.1 | 31.4 vs. 23.4 | 1.13 (0.85–1.50) | 0.40 | – | – | – |
| Pathological fracture | No vs. yes | 45.5 vs. 40.6 | 30.3 vs. 25.4 | 1.05 (0.78–1.43) | 0.74 | – | – | – |
| Pre‐RT ATs | MTs vs. others | 49.0 vs. 43.4 | 28.7 vs. 29.7 | 1.00 (0.77–1.31) | 0.99 | – | – | – |
| Post‐RT ATs | MTs vs. others | 85.3 vs. 24.7 | 68.2 vs. 10.0 | 4.38 (3.32–5.79) | <0.01 | 4.74 (3.42–6.56) | <0.01 | 0.79 |
| Pre‐RT laboratory data | Normal vs. abnormal | 80.8 vs. 41.8 | 57.7 vs. 27.2 | 2.01 (1.27–3.17) | <0.01 | 1.45 (0.83–2.53) | 0.19 | 0.17 |
Abbreviations: ATs, antineoplastic agents; ECOG PS, Eastern Cooperative Oncology Group Performance Status; MTs, molecular‐targeting therapy (tyrosine kinase inhibitors or immune checkpoint inhibitors); NSCLC, non‐small cell lung cancer; RT, radiotherapy; SCLC, small cell lung cancer.
In the multivariate analysis, PS (<3 vs. ≥3; HR, 1.59; 95% CI, 1.22–2.07; p < 0.01), metastases to internal organs (no vs. yes; HR, 1.86; 95% CI, 1.33–2.60; p < 0.01), and post‐RT ATs (MTs vs. others; HR, 4.73; 95% CI, 3.45–6.48; p < 0.01) were significantly associated with reduced OS (Table 2). RT course length (long [≥10 fractions] vs. short [<10 fractions]; HR, 2.27; 95% CI, 1.73–2.98; p < 0.01) was significantly associated with OS in the univariate analysis, but this factor was not included in the multivariate analysis because of selection bias.
Prognosis according to the devised prognostic scoring system
A prognostic scoring system using the regression coefficients of significant prognostic factors in the multivariate analysis was developed (Tables 2 and 3). PS, metastases to internal organs, and post‐RT MTs were used to create a scoring system for the estimation of survival. Based on the regression coefficient (<0.4 = 1 point; ≥0.4, <0.8 = 2 point), the following scoring points were assigned: PS ≥3 = 1 point, PS <3 = 0 points, metastases to internal organs = 1 point, no metastases to internal organs = 0 points, no post‐RT MTs = 2 points, and post‐RT MTs = 0 points. The associations between the total points and the 0.5‐ and 1‐year OS rates are listed in Table 4, and the corresponding Kaplan–Meier curves are shown in Figure 2.
TABLE 3.
Points of significant prognostic factors
| Point | ||
|---|---|---|
| ECOG‐PS | 3–4 | 1 |
| 0–2 | 0 | |
| Metastases on internal organs | Yes | 1 |
| No | 0 | |
| Post‐RT ATs | TKIs or ICIs | 0 |
| No TKIs or ICIs | 2 |
Abbreviations: ATs, antineoplastic agents; ECOG PS, Eastern Cooperative Oncology Group Performance Status; ICIs, immune checkpoint inhibitors; RT, radiotherapy; TKIs, tyrosine kinase inhibitors.
TABLE 4.
Associations between total points and 0.5‐ and 1‐year OS rate
| Total points | n | 0.5‐year OS rate (%) | 1‐year OS rate (%) |
|---|---|---|---|
| 0 | 22 | 100 | 90.9 |
| 1 | 55 | 85.4 | 68.5 |
| 2 | 69 | 67.8 | 46.8 |
| 3 | 133 | 28.0 | 10.1 |
| 4 | 89 | 9.4 | 2.7 |
Abbreviation: OS, overall survival.
FIGURE 2.
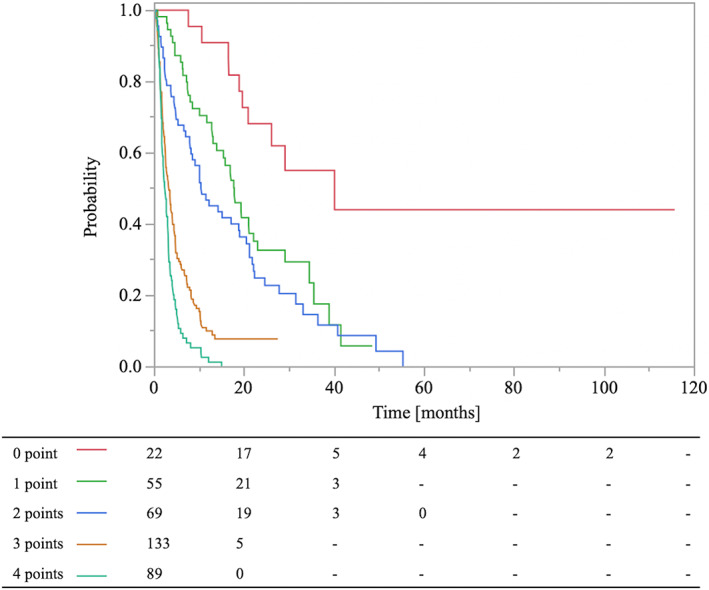
Kaplan–Meier curves of survival rates in lung cancer patients with bone metastases according to different scores.
We classified patients with BMs from LC into three groups, stratified according to our scoring system. The median OS was 25.0 months for the favorable group (total point score 0) (n = 22), 12.8 months for the intermediate group (total point score 1–2) (n = 124), and 2.5 months for the unfavorable group (total point score 3–4) (n = 221). There were statistically significant differences in OS among the three groups (p < 0.01, log‐rank test). The OS curves are shown in Figure 3. In validating this scoring system, the goodness of fit test indicated a statistically significant difference (p < 0.001, χ2 = 25.06). The AUCs for favorable, intermediate, and unfavorable were 0.913, 0.767, and 0.841, respectively.
FIGURE 3.
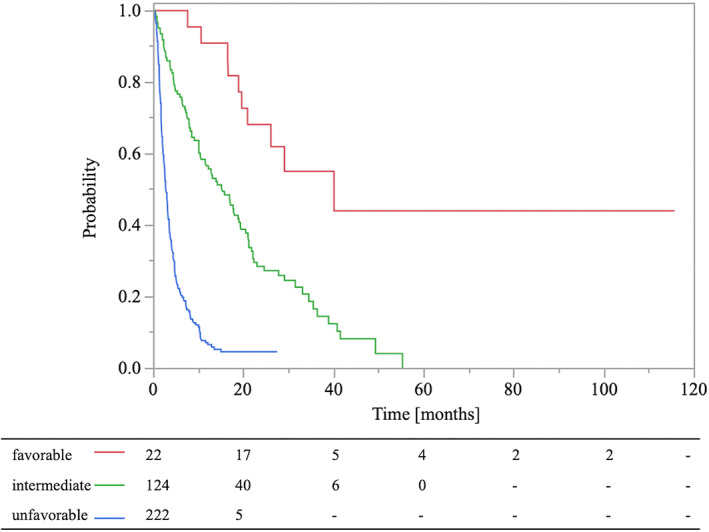
Kaplan–Meier curves of survival rates in lung cancer patients with bone metastases according to total point scores of 0 (favorable), 1–2 (intermediate), and 3–4 (unfavorable).
DISCUSSION
The present study evaluated the prognostic factors for patients who received RT for BMs from LC. Based on our multivariate analysis, PS ≥3, metastases to internal organs, and non‐administration of post‐RT MTs were important unfavorable prognostic factors for survival. Based on these results, patients with BMs from LC were classified into three groups (favorable, intermediate, and unfavorable) according to our scoring system. The median OS was (1) favorable: 2 years, (2) intermediate: 1 year, (3) unfavorable: <3 months.
Katagiri et al.17 suggested a scoring system for predicting BM in patients, which incorporates many factors and is one of the most precise scoring systems. In our study for LC patients with BMs, the factors of the Katagiri scoring system and the latest treatment strategy (ICIs) were evaluated to develop a new LC‐specific scoring system. In our study, ICI administration greatly impacted the prognosis of LC patients with BMs, similar to TKI administration. These two post‐RT treatment strategies appeared to be the most important factor for predicting the prognosis of LC patients with BMs. In contrast, pre‐RT ATs and pre‐RT laboratory data, which were significant factors of the Katagiri scoring system, had little impact on predicting the prognosis of LC patients with BMs. In the Katagiri scoring system, pre‐RT ATs were important because of various cancers being included, rather than LC alone. BMs from breast and prostate cancers, which occurred more frequently, treated with pre‐RT chemotherapy tended to be hormone‐resistant and were often more aggressive. These breast and prostate cancer characteristics influenced the factors of the Katagiri scoring system. Pre‐RT AT administration was not important for predicting prognosis in LC patients with BMs. Meanwhile, in our study, critical abnormal pre‐RT laboratory data were very rare, and the few patients with this data had an extremely poor prognosis (data not shown). Critical abnormal pre‐RT laboratory data were important predictors of extremely poor prognosis, including factors excluded in our scoring system.
Many scoring systems have been suggested for patients with BMs. 14 , 15 , 16 , 17 However, the cohorts of patients in previous studies have included many different types of primary cancer. To consider the individual biology and other individual characteristics of a primary cancer type, specific scoring systems for single cancers are important. In our study, a scoring system for patients with bone metastatic LC with remarkable progress in systemic therapy was devised. Based on our study, only three factors (PS [≥3:1 point], metastases to internal organs [yes:1 point], and post‐RT MTs [no:2 points]) were important for predicting the survival time of patients with bone metastatic LC. In addition, three prognostic groups (favorable: 0 points, intermediate: 1–2 points, and unfavorable: 3–4 points), which were significantly correlated with survival time, were devised according to the regression coefficients of these factors. This easy‐to‐use scoring system would be useful for selecting optimal RT schedules.
In clinical practice, RT with variable dose‐fractionation schedules is administered to BMs for pain relief, prevention of bone fractures, and relief from malignant spinal cord compression. Although a study found that single‐fraction RT was not inferior to multiple‐fraction RT for pain relief in BMs, re‐treatment was more common with single‐fraction RT than with multiple‐fraction RT. 21 Furthermore, differences in the local control of BMs according to dose fractionation schedules have been reported in several studies. 10 , 11 , 12 , 13 Therefore, because hypofractionated low‐dose RT is likely to lead to short‐term control of BMs and is not appropriate for intermediate‐ and long‐term survivors, it should be used in unfavorable patient groups (median OS: <3 months). Moreover, multiple‐fraction RT is likely to lead to intermediate‐term control of BMs and is not appropriate for long‐term survivors; it should, therefore, be used for intermediate groups (median OS: 1 year). In addition, SBRT provides better local control of irradiated bone metastatic sites than conventional RT. 22 Although in oligometastatic patients, SBRT for oligometastatic sites might prolong prognosis. 23 , 24 , 25 Therefore, where possible, SBRT could be used in favorable patients groups (median OS: >2 years).
Our study has several limitations, including its retrospective nature. First, although post‐RT MTs is a future event, this was included as a prognostic factor because post‐RT MTs immediately after RT is available to the radiation oncologist at the start of RT in clinical practice. This had potential risks of selection bias by the attending physician and immortal time bias. However, because this is important information for the radiation oncologist to select the optimal RT dose, this was included in the analysis. In contrast, RT dose schedules (long vs. short) were excluded from the multivariate analysis because of selection bias based on the radiation oncologist. Second, our study analyzed almost all important factors as a reference to the Katagiri scoring system. However, disseminated metastases suggested by the Katagiri scoring system were excluded from our study as LC patients with BMs had disseminated metastases more frequently than metastases to internal organs and had a small prognostic impact (data not shown). Furthermore, because there were few patients with critical abnormal laboratory data, this could not be analyzed in our study. Therefore, further studies are needed to better define an optimal prognostic scoring system for LC patients with BMs. Finally, although internal and external validity of the risk scoring system was assessed in our study, further external validation with other institutional data is also required.
We devised a new scoring system for patients with bone metastatic LC in a new era of targeted therapy. Our easy‐to‐use prognostic model for bone metastatic LC patients would be useful in selecting the appropriate dose‐fractionation schedules. However, updates are required following the progress of systemic therapies.
AUTHOR CONTRIBUTIONS
All authors had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Kenji Makita designed the study concepts. Kenji Makita, Yasushi Hamamoto, Hiromitsu Kanzaki, Kei Nagasaki, and Toshiyuki Kozuki collected patient data and drafted the article. Kenji Makita, Yasushi Hamamoto, Hiromitsu Kanzaki, Kei Nagasaki, and Toshiyuki Kozuki collaborated in the discussion. Kenji Makita and Yasushi Hamamoto prepared the manuscript and Hiromitsu Kanzaki edited the manuscript. All authors read and approved the final manuscript.
CONFLICT OF INTEREST STATEMENT
T.K. received honorarium from MSD, Ono, Kyowa Hakko Kirin, AstraZeneca, Boehringer Ingelheim, Chugai, TAIHO, Eli Lilly, Bristol Myers Squibb, Pfizer, Merck Biopharma, Nippon Kayaku, Novartis, Bayer, Sawai, and AMGEN; consulting fee from Chugai, AstraZeneca, Ono, Pfizer, Daiichi‐Sankyo, Bayer, and AbbVie; and received research funding MSD, Kyowa Hakko Kirin, AstraZeneca, Eli Lilly, Pfizer, Chugai, TAIHO, Ono, Bristol‐Myers, Merck Biopharma, Daiichi‐Sankyo, AbbVie, AMGEN, Sanofi, Eisai, and Labcorp Development. The other authors declare no conflicts of interest.
ACKNOWLEDGMENTS
We thank Ms. Natsumi Yamashita MD, Department of Clinical Research, National Hospital Organization Shikoku Cancer Center, for her statistical support. In addition, we thank Editage (www.editage.jp) for the English language editing.
Makita K, Hamamoto Y, Kanzaki H, Nagasaki K, Kozuki T. An easy tool to predict survival in patients with bone metastatic lung cancer treated with palliative radiotherapy. Thorac Cancer. 2023;14(19):1795–1801. 10.1111/1759-7714.14903
REFERENCES
- 1. Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27:165–76. [DOI] [PubMed] [Google Scholar]
- 2. Tsuya A, Kurata T, Tamura K, Fukuoka M. Skeletal metastases in non‐small cell lung cancer: a retrospective study. Lung Cancer. 2007;57:229–32. [DOI] [PubMed] [Google Scholar]
- 3. Svensson E, Christiansen CF, Ulrichsen SP, Rørth MR, Sørensen HT. Survival after bone metastasis by primary cancer type: a Danish population‐based cohort study. BMJ Open. 2017;7:e016022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for first‐line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378:2288–301. [DOI] [PubMed] [Google Scholar]
- 5. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379:2342–50. [DOI] [PubMed] [Google Scholar]
- 6. Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, et al. First‐line atezolizumab plus chemotherapy in extensive‐stage small‐cell lung cancer. N Engl J Med. 2018;379:2220–9. [DOI] [PubMed] [Google Scholar]
- 7. Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, et al. Overall survival with osimertinib in untreated, EGFR‐mutated advanced NSCLC. N Engl J Med. 2020;382:341–50. [DOI] [PubMed] [Google Scholar]
- 8. Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, et al. First‐line crizotinib versus chemotherapy in ALK‐positive lung cancer. N Engl J Med. 2014;371:2167–77. [DOI] [PubMed] [Google Scholar]
- 9. Chow R, Hoskin P, Schild SE, Raman S, Im J, Zhang D, et al. Single vs multiple fraction palliative radiation therapy for bone metastases: cumulative meta‐analysis. Radiother Oncol. 2019;141:56–61. [DOI] [PubMed] [Google Scholar]
- 10. Chen JJ, Sullivan AJ, Shi DD, Krishnan MS, Hertan LM, Roldan CS, et al. Characteristics and predictors of radiographic local failure in patients with spinal metastases treated with palliative conventional radiation therapy. Adv Radiat Oncol. 2021;6:100665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rades D, Conde‐Moreno AJ, Cacicedo J, Veninga T, Segedin B, Stanic K, et al. 1 × 8 Gy versus 5 × 4 Gy for metastatic epidural spinal cord compression: a matched‐pair study of three prognostic patient subgroups. Radiat Oncol. 2018;13:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Makita K, Hamamoto Y, Kanzaki H, Kataoka M, Yamamoto S, Nagasaki K, et al. Local control of bone metastases treated with external beam radiotherapy in recent years: a multicenter retrospective study. Radiat Oncol. 2021;16:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van der Linden YM, Dijkstra SP, Vonk EJ, Marijnen CA, Leer JW, Dutch Bone Metastasis Study Group . Prediction of survival in patients with metastases in the spinal column: results based on a randomized trial of radiotherapy. Cancer. 2005;103:320–8. [DOI] [PubMed] [Google Scholar]
- 14. Bollen L, van der Linden YM, Pondaag W, Fiocco M, Pattynama BPM, Marijnen CAM, et al. Prognostic factors associated with survival in patients with symptomatic spinal bone metastases: a retrospective cohort study of 1,043 patients. Neuro Oncol. 2014;16:991–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Westhoff PG, de Graeff A, Monninkhof EM, Bollen L, Dijkstra SP, van der Steen‐Banasik E, et al. An easy tool to predict survival in patients receiving radiation therapy for painful bone metastases. Int J Radiat Oncol Biol Phys. 2014;90:739–47. [DOI] [PubMed] [Google Scholar]
- 16. Willeumier JJ, van der Linden YM, van der Wal CWPG, Jutte PC, van der Velden JM, Smolle MA, et al. An easy‐to‐use prognostic model for survival estimation for patients with symptomatic long bone metastases. J Bone Jt Surg Am. 2018;100:196–204. [DOI] [PubMed] [Google Scholar]
- 17. Katagiri H, Okada R, Takagi T, Takahashi M, Murata H, Harada H, et al. New prognostic factors and scoring system for patients with skeletal metastasis. Cancer Med. 2014;3:1359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Braitman LE, Davidoff F. Predicting clinical states in individual patients. Ann Intern Med. 1996;125:406–12. [DOI] [PubMed] [Google Scholar]
- 19. Morgan AG, McAdam WA, Walmsley GL, et al. Clinical findings, early endoscopy, and multivariate analysis in patients bleeding from the upper gastrointestinal tract. BMJ. 1977;2:237–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. [DOI] [PubMed] [Google Scholar]
- 21. Sande TA, Ruenes R, Lund JA, Bruland OS, Hornslien K, Bremnes R, et al. Long‐term follow‐up of cancer patients receiving radiotherapy for bone metastases: results from a randomised multicentre trial. Radiother Oncol. 2009;91:261–6. [DOI] [PubMed] [Google Scholar]
- 22. Zeng KL, Myrehaug S, Soliman H, Husain ZA, Tseng CL, Detsky J, et al. Mature local control and reirradiation rates comparing spine stereotactic body radiation therapy with conventional palliative external beam radiation therapy. Int J Radiat Oncol Biol Phys. 2022;114:293–300. [DOI] [PubMed] [Google Scholar]
- 23. Harrow S, Palma DA, Olson R, Gaede S, Louie AV, Haasbeek C, et al. Stereotactic radiation for the comprehensive treatment of oligometastases (SABR‐COMET): extended long‐term outcomes. Int J Radiat Oncol Biol Phys. 2022;114:611–6. [DOI] [PubMed] [Google Scholar]
- 24. Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR‐comet): a randomised, phase 2, open‐label trial. Lancet. 2019;393:2051–8. [DOI] [PubMed] [Google Scholar]
- 25. Olson R, Mathews L, Liu M, Schellenberg D, Mou B, Berrang T, et al. Stereotactic ablative radiotherapy for the comprehensive treatment of 1‐3 Oligometastatic tumors (SABR‐COMET‐3): study protocol for a randomized phase III trial. BMC Cancer. 2020;20:380. [DOI] [PMC free article] [PubMed] [Google Scholar]


