Abstract
Feral swine are known reservoirs of various pathogens, including Toxoplasma gondii. Here, we report the first national survey of viable T. gondii in feral swine in the USA. We paired serological surveys with parasite isolation and bioassay to evaluate the prevalence and genetic diversity of these parasites. From 2012–2017, sera and tissues from 1517 feral swine across the USA were collected for the isolation of viable T. gondii. Serum samples were initially screened for antibodies to T. gondii, and then the tissues of seropositive feral swine were bioassayed in mice. Antibodies were detected in 27.7% of feral swine tested by the modified agglutination test (1:25 or higher). Antibody positive rates increased significantly with age, with 10.1% of juveniles, 16.0% of sub-adults and 38.4% of adults testing seropositive. Myocardium (50 g) from 232 seropositive feral swine was digested in pepsin and bioassayed in mice. Viable T. gondii was isolated from 78 feral swine from 21 states. Twelve of the 78 isolates were pathogenic to outbred Swiss Webster mice and 76 of the 78 isolates could be propagated further in cell culture and were genotyped. For genotyping, deoxyribonucleic acid extracted from cell culture-derived tachyzoites was characterized by polymerase chain reaction restriction fragment length polymorphism using the genetic markers SAG1, SAG2, SAG3, BTUB, GRA6, c22-8, c29-2, L358, PK1 and Apico. Genotyping revealed 15 ToxoDB genotypes, including 43 isolates for genotype #5 (haplogroup 12), 11 isolates for #24, four isolates for #2 (haplogroup 3), two isolates for each of genotypes #3 (haplogroup 2), #4 (haplogroup 12), #216, #221, #289 and #297 and one isolate for each of genotypes #1 (haplogroup 2), #39, #66, #260, #261 and #299. Genotype #5 was the most frequently isolated, accounted for 57% (43/76) of the isolates, followed by #24, accounted for 14% (11/76). Genotypes #260, #289, #297 and #299 are new types. Genotype #289 was highly virulent to mice and originated from feral swine collected in Louisiana on the same day at the same location. Genotype #216 was previously demonstrated to be highly virulent to mice. Our results indicate moderate genetic diversity of T. gondii in feral swine in the USA, with the genotype #5 (haplogroup 12) dominant in the continental USA, whereas genotype #24 (10/14) was dominant in Hawaii, suggesting different population structures of the parasites among the two distinct geographical locations.
Key words: Feral swine (Sus scrofa), isolation, Toxoplasma gondii, toxoplasmosis, USA
Introduction
The protozoan Toxoplasma gondii infects virtually all warm-blooded animals, including birds, humans, livestock and marine mammals (Dubey, 2010). Domestic pigs are considered important in the epidemiology of toxoplasmosis in the USA (Dubey, 2010), but little is known of the role of feral swine.
Feral swine (Sus scrofa) populations in the USA are estimated to exceed five million and their geographic range continues to expand. Feral swine pose a threat to non-biosecure domestic pig facilities by serving as reservoirs for pathogens which may be transmitted to domestic pigs. In a national survey, antibodies to T. gondii were detected in ~20% of feral swine (Hill et al., 2014). The presence of T. gondii in feral swine is considered a good indicator of contamination in the environment because they are omnivores with a generalist diet, and can become infected by ingesting oocysts while rooting and eating tissues of infected animals. Transmission of T. gondii has been documented in free-ranging domestic pigs through cannibalism (Dubey et al., 1986; Hill et al., 2010). The objective of the present investigation was to isolate and characterize T. gondii from feral swine across the USA.
Materials and methods
Animals and sampled areas
The United States Department of Agriculture's (USDA) Wildlife Services has a task to control feral swine for wildlife damage management purposes and routinely collects sera from a subset for pathogen surveillance. For this study, sera and hearts were collected from 1517 feral swine between September 2012 and October 2017 from 30 states (Table 1). Sex, age (juvenile, sub-adult or adult), date of collection and location information were recorded for each feral swine (Hill et al., 2014). Samples were submitted for T. gondii testing to the USDA's Animal Parasitic Diseases Laboratory in Beltsville, Maryland as described previously (Hill et al., 2014).
Table 1.
Serological prevalence of T. gondii in feral swine collected across the USA from 2012–2017
| Year | Samples received | Male | Female | Juvenile | Sub-adult | Adult | MAT positive (%; 95% CI) | Samples bioassayed | T. gondii isolates |
|---|---|---|---|---|---|---|---|---|---|
| 2012 | 235 | 102 | 133 | 23 | 51 | 161 | 27.6 (22.3–33.7) | 46 | 15 |
| 2013 | 848 | 407 | 439 | 44 | 170 | 634 | 28.5 (25.6–31.7) | 97 | 29 |
| 2014 | 79 | 48 | 31 | 2 | 16 | 61 | 26.6 (18.1–37.2) | 10 | 7 |
| 2015 | 132 | 65 | 66 | 27 | 31 | 72 | 23.5 (17.1–31.4) | 27 | 9 |
| 2016 | 162 | 85 | 77 | 30 | 37 | 95 | 23.5 (17.6–30.1) | 34 | 12 |
| 2017 | 61 | 27 | 34 | 3 | 13 | 45 | 39.3 (28.1–51.9) | 18 | 6 |
| Total | 1517 | 734 | 780 | 129 | 318 | 1068a | 27.7 (27.4–32.1) | 232 | 78 |
Age not recorded for two swine. Sex not recorded for three swine.
Serology
Sera were tested for antibodies to T. gondii by the modified agglutination test (MAT) as described by Dubey and Desmonts (1987). Sera were screened at 1:25, 1:50, 1:100 and 1:200 dilutions or higher.
Isolation by bioassay in mice
A total of 1100 Swiss Webster (SW) mice and 275 INF-γ gene knock-out (KO) mice were used for bioassay and propagation of T. gondii. Myocardium samples (50 g) were homogenized in saline, digested in acidic pepsin, centrifuged and aliquots of homogenates were inoculated subcutaneously into 3–5 outbred albino SW mice, and/or one or two KO mice, which are especially susceptible to toxoplasmosis (Dubey, 2010). Inoculated mice that showed symptoms of toxoplasmosis were terminated and their lungs and brain imprints were examined for T. gondii tachyzoites or tissue cysts, respectively (Dubey, 2010). Survivors were bled 45 days post-inoculation (p.i.) and a 1:25 dilution of serum was tested for T. gondii antibodies by MAT. Mice were euthanized 46 days p.i. and brains of all mice were examined for tissue cysts as described previously (Dubey, 2010). The inoculated mice were considered infected with T. gondii when tachyzoites or tissue cysts were found in their tissues.
Pathogenicity of oocysts of T. gondii strains in mice
To determine mouse pathogenicity of the parasite isolates, four T. gondii isolates showing different virulence levels based on initial observation on bioassay in SW mice were selected. For this, four 3–4 months old T. gondii-free cats (Dubey, 1995) were fed tissues of infected mice. Oocysts collected from the faeces of cats (Dubey, 2010) were sporulated in 2% sulphuric acid for a week on a shaker at room temperature, washed, counted and diluted 10-fold from 10−1 to 10−7 to reach an endpoint of ≅1 oocyst. Aliquots from each dilution of oocysts were fed to each of five SW mice and the recipient mice were examined for T. gondii infection. Mice were examined daily for illness for 2 months, and ill mice were euthanized. Survivors were bled and their sera were tested for T. gondii antibodies and their brains were examined for tissue cysts (Dubey, 2010).
Ethical considerations
All experimental procedures were approved by the Beltsville Area Animal Care and Use Committee (Protocol # 15-017, and 15-018), United States Department of Agriculture. Outbred SW and KO mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA) incompliance with the Institutional Animal Ethics Committee guidelines.
The feral swine were euthanized in the field, often in remote locations, and tissues were transported by the collector to the office, and then shipped by overnight mail. Samples were shipped with ice packs. By the time tissues were received at the USDA laboratory, they often were contaminated with bacteria and not suited for cell culture to isolate T. gondii. A previous study with tissues of naturally infected domestic sows from Iowa indicated that the probability of isolation of T. gondii is very low unless large numbers of mice are used. In this case, of 109 T. gondii isolates obtained from 1000 naturally exposed sows, in most instances only 1 of 10 mice inoculated with sow heart tissue was positive for T. gondii (Dubey et al., 1995). To increase the probability of isolating parasites and minimizing the number of mice, we decided to use five mice for the bioassay of each feral swine in the current study.
All mice and cats used in the present study were treated humanely and examined twice daily for any signs of illness and were supervised by a veterinarian assigned exclusively to the toxoplasmosis project. Any sick mice were euthanized because our objective was isolation of T. gondii and not testing for mortality. We wanted to collect mouse tissues aseptically for cultivation in cell culture or subpassage to other mice. Cats usually do not become ill within 10 days of ingesting T. gondii infected tissues, even though they can excrete many oocysts (Dubey, 2010). In the present study, cats were euthanized 2–3 days after they started excreting T. gondii oocysts.
In vitro cultivation
Infected mouse tissues were seeded onto CV1 cell culture flasks and tachyzoites were harvested from the medium as previously described (Dubey, 2010).
Genotyping of DNA samples
For successful genotyping of T. gondii strains from asymptomatic naturally infected animals, it is necessary to obtain good quality parasite deoxyribonucleic acid (DNA) with minimal contamination of host tissue. Therefore, parasite isolates from mouse tissues were expanded in cell culture. Genotyping of DNA samples by multilocus polymerase chain reaction restriction fragment length polymorphism (PCR-RFLP) markers were carried out following previously reported protocols (Su et al., 2010). Samples with missing data for one to three of the 10 PCR-RFLP markers, but otherwise matching with previously reported genotypes were designated as ‘likely’ of that genotype.
Results
Antibodies to T. gondii were detected in 27.7% (421 of 1517) of feral swine (Table 1). The prevalence of T. gondii antibodies varied only slightly (23.5% to 28.5%) by year from 2012 to 2016. However, seroprevalence was much higher (39.3%) in samples collected in 2017. Among the 734 males and 780 female swine tested, seroprevalence did not differ significantly (26.3% vs 29.2%, χ2 = 1.62 P > 0.20). Seroprevalence increased significantly with age, with 10.1% of juveniles (n = 129), 16.0% of sub-adults (n = 318) and 38.4% of adults (n = 1068) testing positive (χ2 = 78.73, P = 0) (Table 1).
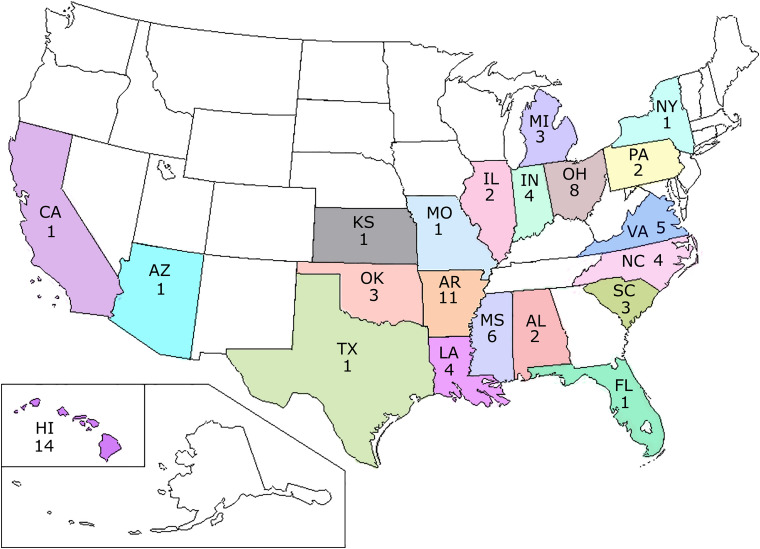
Viable T. gondii was isolated from 78 feral swine from 21 states (Table 2, Fig. 1). The isolation rate increased with MAT titer; parasites were isolated from 11.7% of 17 feral swine with titers of 1:25, from 25% of 20 with titers of 1:50, from 22.6% of 53 with titers of 1:100 and from 39.8% of 143 with titers of ⩾1:200 (Supplementary Table 1).
Table 2.
Toxoplasma gondii isolates from feral swine in the USA from 2012–2017
| Feral pigs | Bioassays in mice | ||||||
|---|---|---|---|---|---|---|---|
| Isolate number | State | County | Collection date | MAT | SW (no. of infected/no. of inoculated) | KO (no. infected/no. inoculated) | Genotype |
| (1) TgFpAL1 | AL | Pike | 4/15/2013 | 400 | 1/3 | ND | #5 |
| (2) TgFpAL2 | AL | Montgomery | 5/17/2013 | 200 | 3/3 | 1/1 | #5 |
| (3) TgFpAR1 | AR | Desha | 10/23/2012 | 50 | 3/4 | 1/1 | #5 |
| (4) TgFpAR2 | AR | Desha | 11/27/2012 | 100 | 2/4 | 1/1 | #5 |
| (5) TgFpAR3 | AR | Desha | 11/27/2012 | 200 | 4/4 | 1/1 | #5 |
| (6) TgFpAR4 | AR | Desha | 11/27/2012 | 200 | 4/4 | 1/1 | #5 |
| (7) TgFpAR5 | AR | Union | 2/19/2013 | 200 | 3/3 | ND | #221 |
| (8) TgFpAR6 | AR | Union | 2/19/2013 | 400 | 1/3 | ND | #5 |
| (9) TgFpAR7 | AR | Union | 2/19/2013 | 200 | 1/3 | ND | #5 |
| (10) TgFpAR8 | AR | Chicot | 3/14/2013 | 200 | 1/3 | ND | #5 |
| (11) TgFpAR9 | AR | Chicot | 3/18/2013 | 200 | 3/3 | ND | #5 |
| (12) TgFpAR10 | AR | Chicot | 3/18/2013 | 200 | 1/3 | ND | #5 |
| (13) TgFpAR11 | AR | Phillips | 5/8/2013 | 200 | 1/3 | ND | #5 |
| (14) TgFpAZ1 | AZ | Mohave | 4/7/2013 | 200 | 3/3 | ND | #5 |
| (15) TgFpCA1 | CA | Nevada | 2/17/2017 | 200 | 2/4 | 1/1 | #66 |
| (16) TgFpFL1 | FL | Pasco | 2/12/2013 | 400 | 1/3 | 1/1 | #221 |
| (17) TgFpHI1 | HI | Honolulu | 10/6/2012 | 200 | 4/4 | 1/1 | #24 |
| (18) TgFpHI2 | HI | Honolulu | 10/15/2012 | 400 | 4/4 | 1/1 | #24 |
| (19) TgFpHI3 | HI | Honolulu | 11/7/2012 | 100 | 3/4 | 1/1 | #24 |
| (20) TgFpHI4 | HI | Honolulu | 11/12/2012 | 50 | 1/4 | 0/1 | #3, Type II |
| (21) TgFpHI5 | HI | Honolulu | 2/9/2013 | 100 | 3/3 | 0/1 | #24 |
| (22) TgFpHI6 | HI | Honolulu | 4/11/2017 | 100 | 5/5 | ND | #24 |
| (23) TgFpHI7 | HI | Kalawao | 2/28/2017 | >200 | 1/4 | 1/1 | #297 (New) |
| (24) TgFpHI8 | HI | Honolulu | 12/19/2013 | 200 | 4/4 | 1/1 | #24 |
| (25) TgFpHI9 | HI | Honolulu | 1/7/2014 | 400 | 3/4 | 1/1 | #260 (New) |
| (26) TgFpHI10 | HI | Honolulu | 1/7/2014 | 100 | 2/4 | 0/1 | #24 |
| (27) TgFpHI11 | HI | Honolulu | 1/14/2014 | >200 | 3/4 | 1/1 | #24 |
| (28) TgFpHI12 | HI | Honolulu | 1/30/2014 | >200 | 1/4 | 1/1 | #261 |
| (29) TgFpHI13 | HI | Honolulu | 2/2/2014 | >200 | 4/4 | 1/1 | #24 |
| (30) TgFpHI14 | HI | Honolulu | 2/11/2014 | 1600 | 2/5 | ND | #24 |
| (31) TgFpIL1 | IL | Du Page | 10/20/2016 | 200 | 0/4 | 1/1 | #1, Type II |
| (32) TgFpIL2 | IL | Fulton | 5/23/2013 | 200 | 2/3 | 2/2 | #3, Type II |
| (33) TgFpIN1 | IN | Lawrence | 12/23/2012 | 100 | 0/4 | 1/1 | #39 |
| (34) TgFpIN2 | IN | Lawrence | 10/15/2015 | >200 | 1/4 | 1/1 | #2 likely |
| (35) TgFpIN3 | IN | Washington | 11/3/2015 | 200 | 1/4 | 1/1 | #216 likely |
| (36) TgFpIN4 | IN | Lawrence | 4/12/2017 | 25 | 0/4 | 1/1 | #4 |
| (37) TgFpKS1 | KS | Bourbon | 3/27/2013 | 200 | 2/3 | ND | #5 |
| (38) TgFpLA1 | LA | Orleans | 11/6/2012 | 400 | 4/4 | 1/1 | #289, New |
| (39) TgFpLA2 | LA | Orleans | 11/6/2012 | 3200 | 2/4 | 1/1 | #289, New |
| (40) TgFpLA3 | LA | East Feliciana | 3/6/2013 | 200 | 1/3 | ND | ND |
| (41) TgFpLA4 | LA | West Feliciana | 3/7/2013 | 1600 | 1/3 | ND | #5 |
| (42) TgFpMI1 | MI | Bay | 10/29/2015 | 200 | 4/4 | 1/1 | #5 likely |
| (43) TgFpMI2 | MI | Marquette | 11/12/2015 | >200 | 4/4 | 1/1 | #5 |
| (44) TgFpMI3 | MI | Midland | 2/2/2016 | 50 | 4/4 | 1/1 | #299 (New) |
| (45) TgFpMO1 | MO | Reynolds | 9/19/2012 | 100 | 3/4 | 1/1 | #5 |
| (46) TgFpMS1 | MS | Yazoo | 10/1/2012 | 200 | 4/4 | 1/1 | #5 |
| (47) TgFpMS2 | MS | Sharkey | 1/3/2013 | 100 | 4/4 | 1/1 | #5 |
| (48) TgFpMS3 | MS | Yazoo | 3/22/2013 | 400 | 3/3 | ND | #5 |
| (49) TgFpMS4 | MS | Yazoo | 3/26/2013 | 800 | 3/3 | ND | #5 |
| (50) TgFpMS5 | MS | Yazoo | 3/26/2013 | 200 | 3/3 | ND | #5 |
| (51) TgFpMS6 | MS | Yazoo | 5/28/2013 | 200 | 1/3 | 1/2 | #5 |
| (52) TgFpNC1 | NC | Bladen | 10/16/2012 | 400 | 0/4 | 1/1 | ND |
| (53) TgFpNC2 | NC | Bladen | 7/28/2015 | 200 | 4/4 | 1/1 | #5 likely |
| (54) TgFpNC3 | NC | Columbus | 8/3/2015 | >200 | 3/4 | 0/1 | #5 likely |
| (55) TgFpNC4 | NC | Duplin | 4/13/2016 | 50 | 4/4 | 1/1 | #5 likely |
| (56) TgFpNY1 | NY | Clinton | 8/21/2013 | 800 | 2/3 | 1/1 | #5 |
| (57) TgFpOH1 | OH | Lorain | 11/21/2013 | 400 | 1/4 | 0/1 | #4 |
| (58) TgFpOH2 | OH | Jackson | 5/25/2016 | 100 | 4/4 | 1/1 | #24 |
| (59) TgFpOH3 | OH | Gallia | 8/10/2016 | 200 | 1/4 | 1/1 | #2 |
| (60) TgFpOH4 | OH | Gallia | 8/10/2016 | 100 | 2/4 | 0/1 | #297 (New) |
| (61) TgFpOH5 | OH | Gallia | 10/20/2016 | 200 | 1/4 | 1/1 | #5 |
| (62) TgFpOH6 | OH | Gallia | 10/20/2016 | 200 | 4/4 | 1/1 | #2 likely |
| (63) TgFpOH7 | OH | Gallia | 10/20/2016 | 400 | 0/4 | 1/1 | #5 likely |
| (64) TgFpOH8 | OH | Gallia | 10/20/2016 | 200 | 3/4 | 1/1 | #5 |
| (65) TgFpOK1 | OK | Tillman | 1/14/2013 | 100 | 3/4 | 1/1 | #5 |
| (66) TgFpOK2 | OK | Tillman | 1/14/2013 | 400 | 2/4 | 1/1 | #5 |
| (67) TgFpOK3 | OK | Choctaw | 3/8/2013 | 800 | 1/3 | ND | #5 |
| (68) TgFpPA1 | PA | Fulton | 3/12/2016 | 25 | 3/4 | 0/1 | #5 likely |
| (69) TgFpPA2 | PA | Bedford | 4/27/2016 | 800 | 4/4 | 1/1 | #5 likely |
| (70) TgFpSC1 | SC | Georgetown | 3/5/2014 | >200 | 2/5 | ND | #5 likely |
| (71) TgFpSC2 | SC | Georgetown | 1/25/2013 | 400 | 1/3 | 1/1 | #5 |
| (72) TgFpSC3 | SC | Georgetown | 3/6/2013 | 400 | 1/3 | ND | #5 |
| (73) TgFpTX1 | TX | Hemphill | 12/3/2012 | 100 | 1/4 | 1/1 | #5 |
| (74) TgFpVA1 | VA | Lee | 6/14/2015 | >200 | 1/4 | 0/1 | #5 likely |
| (75) TgFpVA2 | VA | Culpeper | 10/1/2015 | 25 | 4/4 | 1/1 | #2 likely |
| (76) TgFpVA3 | VA | Lee | 12/18/2015 | 100 | 1/4 | 0/1 | #216 |
| (77) TgFpVA4 | VA | Chesapeake City | 4/10/2017 | >200 | 3/4 | 1/1 | #5 |
| (78) TgFpVA5 | VA | Chesapeake City | 4/10/2017 | 50 | 2/4 | 1/1 | #5 |
AL, Alabama; AR, Arkansas; AZ, Arizona; CA, California; FL, Florida; HI, Hawaii; IL, Illinois; IN, Indiana; KS, Kansas; LA, Louisiana; MI, Michigan; MO, Missouri; MS, Mississippi; NC, North Carolina; NY, New York; OH, Ohio; OK, Oklahoma; PA, Pennsylvania; SC, South Carolina; TX, Texas; VA, Virginia; SW, Swiss Webster albino mice; KO, Interferon-γ Knockout mice; ND, Not done.
Fig. 1.
Map of USA showing the T. gondii isolates from feral swine.
The SW mice inoculated with tissue digest of hearts from 12 of the 78 infected feral swine showed symptoms of T. gondii infection and a few died or were euthanized between 11 and 27 days p.i. (Table 3).
Table 3.
Isolates of pathogenic T. gondii identified in feral swine collected across the USA from 2012–2017
| Isolate number | Feral pig ID | Collection date | State | County | Age class | Sex | MAT | Bioassayed in SW micea | Genotype | |
|---|---|---|---|---|---|---|---|---|---|---|
| No. of mice infected with T. gondii | No. of mice died/euthanized (day) | |||||||||
| 15 TgFpCA1 | ID0034613 | 2/17/2017 | CA | Nevada | Sub-Adult | Male | 200 | 2 | 1 (26) | #66 |
| 17 TgFpHI1 | ID0016782 | 10/6/2012 | HI | Honolulu | Sub-Adult | Female | 200 | 4b | 1 (11) | #24 |
| 26 TgFpHI10 | ID0019838 | 1/7/2014 | HI | Honolulu | Adult | Male | 100 | 2 | 1 (19) | #24 |
| 27 TgFpHI11 | ID0019841 | 1/14/2014 | HI | Honolulu | Adult | Male | >200 | 3 | 1 (23) | #24 |
| 35 TgFpIN3 | ID0030648 | 11/3/2015 | IN | Washington | Adult | Female | 200 | 1c | 1 (14) | #216 Likely |
| 38 TgFpLA1 | ID0017224 | 11/6/2012 | LA | Orleans | Adult | Female | 400 | 4b | 4 (15, 20, 20, 22) | #289, New |
| 39 TgFpLA2 | ID0017225 | 11/6/2012 | LA | Orleans | Adult | Female | 3200 | 2 | 2 (20, 20) | #289, New |
| 40 TgFpLA3 | ID0017282 | 3/6/2013 | LA | East Feliciana | Adult | Male | 200 | 1 | 1 (12) | Not done |
| 46 TgFpMS1 | ID0017303 | 10/1/2012 | MS | Yazoo | Adult | Female | 200 | 1b | 1 (14) | #5 |
| 54 TgFpNC3 | ID0026419 | 8/3/2015 | NC | Columbus | Adult | Male | >200 | 3 | 1 (27) | #5 Likely |
| 75 TgFpVA3 | ID0031871 | 12/18/2015 | VA | Lee | Adult | Male | 100 | 1 | 1 (15) | #216 |
| 76 TgFpVA4 | ID0031573 | 4/10/2017 | VA | Chesapeake City | Adult | Female | >200 | 3 | 1 (19) | #5 |
Four mice were inoculated with pig hearts.
One SW mouse from the group fed to cat.
Three out of four mice died on day 2 – it was no due to toxoplasmosis.
The four cats fed infected mice excreted T. gondii oocysts but remained clinically healthy and were euthanized in good health 7–10 days after feeding infected mouse tissues. Oocysts of two isolates (TgFpLA1 and TgFpLA2, both are genotype #289) were very pathogenic to SW mice; all mice fed their oocysts died/or euthanized of acute toxoplasmosis enteritis or pneumonia and tachyzoites were found in lungs of all infected mice (Table 4). The isolate TgFpHI1 (genotype #24) was mildly pathogenic; mice fed 100 oocysts had signs of acute toxoplasmosis whereas mice fed fewer than 100 oocysts survived and remained asymptomatic. For isolate TgFpMS1 (genotype #5), only a few oocysts were present and low doses (10 and 1 oocysts) were used to challenge mice, all infected mice survived (Table 4).
Table 4.
Pathogenicity of oocysts of four T. gondii isolates derived from feral swine
| Dosea | Isolate number (ToxoDB genotype) | |||
|---|---|---|---|---|
| TgFpMS1 (#5) | TgFpHI1 (#24) | TgFpLA1 (#289) | TgFpLA2 (#289) | |
| 100 | Not done | 5 (10, 10, 10, 12, 15) | 5 (9, 9, 9, 9, 9) | 5 (8, 8, 9, 9, 9) |
| 10 | 5 (S, S, S, S, S)b | 4 (S, S, S, S) | 3 (10, 10, 13) | 5 (8, 9, 9, 9, 12) |
| 1 | 5 (S, S, S, S, S) | 2 (S, 16) | 1 (13) | 5 (9, 12, 12, 12, 12) |
| <1 | 0 | Not done | 0 | 0 |
S, Survived, infected with T. gondii.
Five mice per group. Oocysts were inoculated orally.
Based-on estimation that the last infective dilution has one infective organism.
No. of mice dead, and day of death in parenthesis.
Seventy-six of the 78 isolates were genotyped (Table 5); typing results for individual isolates are shown in Supplementary Table 2. The results revealed 15 ToxoDB genotypes, including 43 isolates for genotype #5 (haplogroup 12), 11 isolates for #24, four isolates for genotype #2 (haplogroup 3), two isolates for each of genotypes #3 (haplogroup 2), #4 (haplogroup 12), #216, #221, #289 and #297 and one isolate for each of genotypes #1 (haplogroup 2), #39, #66 (haplogroup 11), #260, #261 and #299. Genotype #5 was the most frequently isolated, accounted for 57.5% (43/76) of the isolates, followed by #24, accounting for 14% (11/76). Genotypes #260, #289, #297 and #299 have not been previously reported. Genotype #289 was mouse-virulent, and originated from each of two feral swine collected concurrently from a location in Louisiana; no other information was available regarding these pigs.
Table 5.
Toxoplasma gondii isolates and genotype by State of feral swine collected from 2012–2017
| State | Samples bioassayed | No. of isolates | Toxo DB genotype |
|---|---|---|---|
| AL | 10 | 2 | #5 |
| AR | 26 | 11 | #5; #221 |
| AZ | 1 | 1 | #5 |
| CA | 3 | 1 | #66 |
| FL | 7 | 1 | #221 |
| HI | 28 | 14 | #24; #3 Type II; #260 (New); #261; #297 (New) |
| IL | 2 | 2 | #1 Type II; #3 Type II |
| IN | 9 | 4 | #39; #2 likely; #4; #216 likely |
| KS | 18 | 1 | #5 |
| LA | 10 | 4 | #289 (New); #5 |
| MI | 10 | 3 | #5 likely; #1 or #3 Type II; #299 (New) |
| MO | 14 | 1 | #5 |
| MS | 14 | 6 | #5 |
| NC | 14 | 4 | #5 likely |
| NY | 1 | 1 | #5 |
| OH | 16 | 8 | #4; #24; #2; #2 likely; #5 likely; #5; 297 (New) |
| OK | 3 | 3 | #5 |
| PA | 9 | 2 | #5 likely |
| SC | 5 | 3 | #5 likely; #5 |
| TX | 6 | 1 | #5 |
| VA | 13 | 5 | #5 likely; #2 likely; #5; #216 |
| GA | 2 | 0 | |
| Guan | 3 | 0 | |
| KY | 1 | 0 | |
| NV | 1 | 0 | |
| TN | 4 | 0 | |
| UT | 2 | 0 | |
| Total | 232 | 78 | #1 (n = 1), #2 (n = 4), #3 (n = 2), #4 (n = 2), #5 (n = 43), #24 (n = 11), #39 (n = 1), #66 (n = 1), #216 (n = 2), #221 (n = 2), #260 (n = 1), #261 (n = 1), #289 (n = 2), #297 (n = 2), #299 (n = 1) |
Two of the 78 isolates were not typed.
Discussion
The primary objective of the present study was to isolate and genetically characterize T. gondii occurring in feral swine in the USA. We have previously reported seroprevalence of T. gondii in feral swine samples collected between 2006–2013, which was 17.7% by ELISA and 28.4% by MAT (Hill et al., 2014). The results of this study (27.7% seroprevalence) supplement previously reported data and indicate that T. gondii infection remains high in feral swine in the USA. This prevalence of T. gondii antibodies was like the 23% (4759 of 16 788) seroprevalence detected in wild pigs worldwide (Rostami et al., 2017).
Most isolates from feral swine in the present study were identified as ToxoDB genotype #5, which is the dominant type in North American wildlife (Dubey et al., 2011; Khan et al., 2011; Jiang et al., 2018). This contrasts with what has been most frequently derived from domestic pigs in USA, in which the dominant T. gondii genotypes are #1 and #3 (collectively known as type II, haplogroup 2) and #3 (type III, haplogroup 3) (Velmurugan et al., 2009; Jiang et al., 2018). Importantly, these data substantiate a distinction between transmission among feral and farmed pigs in North America (Jiang et al., 2018). In Europe, the type II T. gondii lineage is dominant in human population, and genotyping data showed that it is also true in wildlife including wild hogs (Richomme et al., 2009; Aubert et al., 2010), suggesting T. gondii population is largely homogeneous and no partition of parasite genotypes in the region. Several studies in China indicated dominance of ToxoDB genotype #9 in domestic pigs (Zhou et al., 2010; Jiang et al., 2013; Wang et al., 2016), however, there is limited information regarding genotypes in wildlife for a comparison. Recent data from Brazil indicated high genetic diversity of T. gondii in domestic pigs (Feitosa et al., 2017). But information is still limited to compare the parasites from domestic animal vs wildlife. To better understand the partition of transmission, studies of T. gondii genotypes in domestic animals and wildlife in other regions such as Asia, Africa, Australia and South America are needed.
Pathogenicity of oocysts of four T. gondii strains in mice suggested that the newly identified genotype #289 (isolates TgFpLA1 and TgFpLA2) is highly virulent. However, genotype #24 (isolate TgFpHI1), common in Hawaii, is mildly pathogenic. The genotype #5 (isolate TgFpMS1), prevalent in wildlife in North America, is also mildly pathogenic to mice. This result indicates that, even though most T. gondii strains in the U.S. are not highly virulent, there is a low frequency of highly virulent parasites circulating in wildlife.
Genotype #24 was the second-most frequently isolated type in this study. Ten of the 14 isolates identified in Hawaii belong to #24, which accounted for 71% (10/14) of those isolates (Table 2). Genotype #24 has previously been identified in chickens from Costa Rica and Brazil (Dubey et al., 2006; Ferreira et al., 2018), and in bobcats from Mississippi, USA (Verma et al., 2017), suggesting it is widely distributed in the USA. Bioassays in mice indicate #24 strains are not highly virulent to mice (Tables 3 and 4). Among the four new genotypes identified in this study, two (#260 and #297) were from Hawaii. In addition, genotype #261 was also first identified in Hawaiian geese (Work et al., 2016). These results indicate that the T. gondii population in Hawaii differs from those in the continental USA.
Other genotypes, including #1, #2, #3, #4, #39 #66, #216 and #221, have previously been identified in animals in the USA, with the first four being common (Jiang et al., 2018). Among these genotypes, #216 is highly virulent to mice (Dubey et al., 2013a, 2013b, 2015).
Recent evidence indicates that wildlife T. gondii strains can also cause clinical disease in humans (Jokelainen et al., 2018; Pomares et al., 2018) and domestic cats (Dubey and Prowell, 2013; Crouch et al., 2019). It is suggested that partition of T. gondii genotypes among domestic animals and wildlife is mainly due to distinct sylvatic and domestic transmission cycles, though both cycles overlap to a certain degree (Shwab et al., 2018).
Our results revealed moderate genetic diversity of T. gondii in feral swine in the USA, with genotype #5 (haplogroup 12) dominant in continental USA, whereas genotype #24 (10/14) was dominant in Hawaii, suggesting different population structures of the parasites among the two distinct genographical locations. The T. gondii isolates detected in feral swine generally resembled those found in other wildlife species and were distinct from those that are typically identified in domestic pigs, and include novel genotypes including ones that are highly virulent to mice. The contribution of feral swine as a reservoir of infection deserves additional scrutiny, as well as their potential in disseminating parasites to humans.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
None.
Ethical standards
Feral swine were sampled following protocols developed by WS' National Wildlife Disease Program (NWDP) (USDA-APHIS-WS, NWDP, Comprehensive Feral Swine Disease Surveillance Procedure Manual, 2012).
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0031182019001586.
click here to view supplementary material
References
- Aubert D, Ajzenberg D, Richomme C, Gilot-Fromont E, Terrier ME, de Gevigney C, Game Y, Maillard D, Gibert P, Dardé ML and Villena I (2010) Molecular and biological characteristics of Toxoplasma gondii isolates from wildlife in France. Veterinary Parasitology 171, 346–349. [DOI] [PubMed] [Google Scholar]
- Crouch EEV, Mittel LD, Southard TL, Cerqueira-Cézar CK, Murata FHA, Kwok OCH, Su C and Dubey JP (2019) Littermate cats rescued from a shelter succumbed to acute, primary toxoplasmosis associated with TOXO DB genotype #4, generally circulating in wildlife. Parasitology International 72, article 101942. [DOI] [PubMed] [Google Scholar]
- Dubey JP (1995) Duration of immunity to shedding of Toxoplasma gondii oocysts by cats. Journal of Parasitology 81, 410–415. [PubMed] [Google Scholar]
- Dubey JP (2010) Toxoplasmosis of Animals and Humans, 2nd Edn. Boca Raton, Florida, USA: CRC Press, pp. 1–313. [Google Scholar]
- Dubey JP and Desmonts G (1987) Serological responses of equids fed Toxoplasma gondii oocysts. Equine Veterinary Journal 19, 337–339. [DOI] [PubMed] [Google Scholar]
- Dubey JP and Prowell M (2013) Ante-mortem diagnosis, diarrhea, oocyst shedding, treatment, isolation and genetic typing of Toxoplasma gondii associated with clinical toxoplasmosis in a naturally-infected cat. Journal of Parasitology 99, 158–160. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Murrell KD, Hanbury RD, Anderson WR, Doby PB and Miller HO (1986) Epidemiologic findings on a swine farm with enzootic toxoplasmosis. Journal of the American Veterinary Medical Association 189, 55–56. [PubMed] [Google Scholar]
- Dubey JP, Thulliez P and Powell EC (1995) Toxoplasma gondii in Iowa sows: comparison of antibody titers to isolation of T. gondii by bioassays in mice and cats. Journal of Parasitology 81, 48–53. [PubMed] [Google Scholar]
- Dubey JP, Su C, Oliveira J, Morales JA, Bolaños RV, Sundar N, Kwok OCH and Shen SK (2006) Biologic and genetic characteristics of Toxoplasma gondii isolates in free-range chickens from Costa Rica, Central America. Veterinary Parasitology 139, 29–36. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Velmurugan GV, Rajendran C, Yabsley MJ, Thomas NJ, Beckmen KB, Sinnett D, Ruid D, Hart J, Fair PA, McFee WE, Shearn-Bochsler V, Kwok OCH, Ferreira LR, Choudhary S, Faria EB, Zhou H, Felix TA and Su C (2011) Genetic characterisation of Toxoplasma gondii in wildlife from North America revealed widespread and high prevalence of the fourth clonal type. International Journal for Parasitology 41, 1139–1147. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Choudhary S, Kwok OCH, Ferreira LR, Oliveira S, Verma SK, Marks DR, Pedersen K, Mickley RM, Randall AR, Arsnoe D and Su C (2013a) Isolation and genetic characterization of Toxoplasma gondii from mute swan (Cyngus olor) from the USA. Veterinary Parasitology 195, 42–46. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Hill D, Zarlenga D, Choudhary S, Ferreira LR, Oliveira S, Verma SK, Kwok OCH, Driscoll CP, Spiker H and Su C (2013b) Isolation and characterization of new genetic types of Toxoplasma gondii and prevalence of Trichinella murrelli from black bear (Ursus americanus). Veterinary Parasitology 196, 24–30. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Verma SK, Calero-Bernal R, Cassinelli AB, Kwok OCH, van Why K, Su C and Humphreys JG (2015) Isolation and genetic characterization of Toxoplasma gondii from black bears (Ursus americanus), bobcats (Lynx rufus), and feral cats (Felis catus) from Pennsylvania. The Journal of Eukaryotic Microbiology 62, 410–415. [DOI] [PubMed] [Google Scholar]
- Feitosa TF, Vilela VLR, de Almeida-Neto JL, dos Santos A, de Morais DF, Alves BF, Nakashima F, Gennari SM, Athayde ACR and Pena HFJ (2017) High genetic diversity in Toxoplasma gondii isolates from pigs at slaughterhouses in Paraíba state, northeastern Brazil: circulation of new genotypes and Brazilian clonal lineages. Veterinary Parasitology 224, 76–80. [DOI] [PubMed] [Google Scholar]
- Ferreira TCR, Buery JC, Moreira NIB, Santos CB, Costa JGL, Pinto LV, Baraviera RCA, Vitor RWA and Fux B (2018) Toxoplasma gondii: isolation, biological and molecular characterisation of samples from free-range Gallus gallus domesticus from countryside Southeast Brazil. Brazilian Journal of Veterinary Parasitology 27, 384–389. [DOI] [PubMed] [Google Scholar]
- Hill DE, Haley C, Wagner B, Gamble HR and Dubey JP (2010) Seroprevalence of and risk factors for Toxoplasma gondii in the US swine herd using sera collected during the National Animal Health Monitoring Survey (Swine 2006). Zoonoses and Public Health 57, 53–59. [DOI] [PubMed] [Google Scholar]
- Hill DE, Dubey JP, Baroch JA, Swafford SR, Fournet VF, Hawkins-Cooper D, Pyburn DG, Schmit BS, Gamble HR, Pedersen K, Ferreira LR, Verma SK, Ying Y, Kwok OC, Feidas H and Theodoropoulos G (2014) Surveillance of feral swine for Trichinella spp. and Toxoplasma gondii in the USA and host-related factors associated with infection. Veterinary Parasitology 205, 653–665. [DOI] [PubMed] [Google Scholar]
- Jiang HH, Huang SY, Zhou DH, Zhang XX, Su C, Deng SZ and Zhu XQ (2013) Genetic characterization of Toxoplasma gondii from pigs from different localities in China by PCR-RFLP. Parasites & Vectors 6, 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T, Shwab EK, Martin RM, Gerhold RW, Rosenthal BM, Dubey JP and Su C (2018) A partition of Toxoplasma gondii genotypes across spatial gradients and among host species, and decreased parasite diversity towards areas of human settlement in North America. International Journal for Parasitology 48, 611–619. [DOI] [PubMed] [Google Scholar]
- Jokelainen P, Murat JB and Nielsen HV (2018) Direct genetic characterization of Toxoplasma gondii from clinical samples from Denmark: not only genotypes II and III. European Journal of Clinical Microbiology and Infectious Diseases 37, 579–586. [DOI] [PubMed] [Google Scholar]
- Khan A, Dubey JP, Su C, Ajioka JW, Rosenthal BM and Sibley LD (2011) Genetic analyses of atypical Toxoplasma gondii strains reveals a fourth clonal lineage in North America. International Journal for Parasitology 41, 645–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomares C, Devillard S, Holmes TH, Olariu TR, Press CJ, Ramirez R, Talucod J, Estran R, Su C, Dubey JP, Ajzenberg D and Montoya JG (2018) Genetic characterization of Toxoplasma gondii DNA samples isolated from humans living in North America: an unexpected high prevalence of atypical genotypes. Journal of Infectious Diseases 218, 1783–1791. [DOI] [PubMed] [Google Scholar]
- Richomme C, Aubert D, Gilot-Fromont E, Ajzenberg D, Mercier A, Ducrot C, Ferté H, Delorme D and Villena I (2009) Genetic characterization of Toxoplasma gondii from wild boar (Sus scrofa) in France. Veterinary Parasitology 164, 296–300. [DOI] [PubMed] [Google Scholar]
- Rostami A, Riahi SM, Fakhri Y, Saber V, Hanifehpour H, Valizadeh S, Gholizadeh M, Pouya RH and Gamble HR (2017) The global seroprevalence of Toxoplasma gondii among wild boars: a systematic review and meta-analysis. Veterinary Parasitology 244, 12–20. [DOI] [PubMed] [Google Scholar]
- Shwab EK, Saraf P, Zhu XQ, Zhou DH, McFerrin BM, Ajzenberg D, Schares G, Hammond-Aryee K, van Helden P, Higgins SA, Gerhold RW, Rosenthal BM, Zhao X, Dubey JP and Su C (2018) Human impact on the diversity and virulence of the ubiquitous zoonotic parasite Toxoplasma gondii. Proceedings of the National Academy of Sciences of the United States of America 115, E6956–E6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su C, Shwab EK, Zhou P, Zhu XQ and Dubey JP (2010) Moving towards an integrated approach to molecular detection and identification of Toxoplasma gondii. Parasitology 137, 1–11. [DOI] [PubMed] [Google Scholar]
- Velmurugan GV, Su C and Dubey JP (2009) Isolate designation and characterization of Toxoplasma gondii isolates from pigs in the United States. Journal of Parasitology 95, 95–99. [DOI] [PubMed] [Google Scholar]
- Verma SK, Sweeny AR, Lovallo MJ, Calero-Bernal R, Kwok OC, Jiang T, Su C, Grigg ME and Dubey JP (2017) Seroprevalence, isolation, and co-infection of multiple Toxoplasma gondii strains in individual bobcats (Lynx rufus) from Mississippi, USA. International Journal for Parasitology 47, 297–303. [DOI] [PubMed] [Google Scholar]
- Wang D, Liu Y, Jiang T, Zhang G, Yuan G, He J, Su C and Yang N (2016) Seroprevalence and genotypes of Toxoplasma gondii isolated from pigs intended for human consumption in Liaoning province, northeastern China. Parasites & Vectors 9, 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Work TM, Verma SK, Su C, Medeiros J, Kaiakapu T, Kwok OC and Dubey JP (2016) Toxoplasma gondii antibody prevalence and two new genotypes of the parasite in endangered Hawaiian geese (nene: Branta sandvicensis). Journal of Wildlife Diseases 52, 253–257. [DOI] [PubMed] [Google Scholar]
- Zhou P, Nie H, Zhang LX, Wang HY, Yin CC, Su C, Zhu XQ and Zhao JL (2010) Genetic characterization of Toxoplasma gondii isolates from pigs in China. Journal of Parasitology 96, 1027–1029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0031182019001586.
click here to view supplementary material