Abstract
Bovine trypanosomosis has been spreading in Brazil. In the present study, we evaluated the spatial distribution, prevalence and risk factors of this disease in the state of Goiás, Brazil, and performed both molecular and phylogenetical analyses of Trypanosoma vivax. A total of 4049 blood samples were collected from cattle for a period of 2 years. The parasitological diagnosis was performed using the Woo method and a questionnaire was administered to the farmers to document risk factors associated with the disease in the herd. Positive samples were DNA sequenced and compared to GenBank codes. The prevalence of T. vivax was 8.84%, occurring on 24 ranches only in dairy cattle and mainly in the central and southern portions of the state. The acquisition of new animals infected with T. vivax and the administration of exogenous oxytocin to cows using the same syringe and needle were the main associated factors (P ≤ 0.05). After an outbreak, milk production decreased by 39.62%. The presence of biting flies (tabanids, Haematobia irritans and Stomoxys calcitrans) was not a risk factor (P > 0.05) for the occurrence of T. vivax. The epidemiological data demonstrate the importance of restricting the practice of auctions as well as eliminating the use of exogenous oxytocin in animals during milking. The samples tested by polymerase chain reaction were positive for T. vivax and were genetically homologous with T. vivax found in different states of Brazil and west Africa based on the 18S rRNA gene.
Key words: Biting flies, epidemiology, iatrogenic route, molecular characterization, risk factors, trypanosomosis
Introduction
Trypanosoma vivax causes trypanosomosis in cattle. This haemoprotozoan originating from Africa is believed to have been introduced in South America around the year 1830, with the transport of infected cattle from Senegal (Ventura et al., 2001; Osório et al., 2008). In sub-Saharan Africa, the transmission of this protozoa occurs biologically through the tsetse fly (Glossina spp.). In Central and South America, transmission occurs mechanically by biting flies (tabanids, Stomoxys calcitrans and Haematobia irritans) or by iatrogenic means (Cadioli et al., 2012; Dagnachew and Bezie, 2015; Bastos et al., 2017).
The first outbreak of trypanosomosis in Brazil occurred in the state of Pará in 1972 (Shaw and Lainson, 1972). From the year 2000 to 2017, occcurences have been reported in nearly 60% of the states of the country: Mato Grosso do Sul, Mato Grosso, Paraíba, Maranhão, Tocantins, Minas Gerais, São Paulo, Rio Grande do Sul, Pernambuco, Alagoas, Goiás, Sergipe, Piauí, Rio de Janeiro and Rio Grande do Norte (Silva et al., 1996; Paiva et al., 2000; Linhares et al., 2006; Batista et al., 2007; Carvalho et al., 2008; Guerra et al., 2008; Silva et al., 2009; Cadioli et al., 2012; Pimentel et al., 2012; Andrade Neto et al. 2015; Costa et al., 2016; Bastos et al., 2017; Vieira et al., 2017; Lopes et al., 2018).
In the present study, we evaluated the spatial distribution, prevalence and risk factors of the acute occurrence of trypanosomosis in cattle in the state of Goiás, Brazil, and performed molecular analyses for the identification of T. vivax during an outbreak, with comparisons to previous findings based on the 18S rRNA gene.
Materials and methods
Ethics statement
This study received approval from the Animal Use Ethics Committee of the Federal University of Goiás, Brazil (certificate number: 032/15) and was conducted in compliance with the ethical principles governing animal experimentation of the Brazilian National Animal Experimentation Control Council (CONCEA).
Properties and animals for the study of T. vivax
The properties registered with the Agriculture and Livestock Defense Agency of the state of Goiás (AGRODEFESA-GO) that did not receive any medication with specific action against T. vivax, had cattle with acute problems suggestive of trypanosomosis and reported recent animal deaths were selected for visits between May 2015 and May 2017. The owners were contacted by phone for visit arrangements.
Forty-two ranches were visited in 26 cities in the state of Goiás: Alexânia, Anápolis, Bonfinópolis, Buriti Alegre, Caldas Novas, Campo Alegre de Goiás, Corumbaíba, Cromínia, Edealina, Gameleira de Goiás, Goianápolis, Goianésia, Goiatuba, Guapó, Ipameri, Itaberaí, Itauçu, Jataí, Mairipotaba, Mambaí, Morrinhos, Pontalina, Porteirão, Quirinópolis, Santa Bárbara de Goiás and Urutaí. A total of 4049 blood samples were collected from cattle. Approximately 73% of the bovines evaluated were the Girolando breed (1/2 Holstein + 1/2 Gyr and 3/4 Holstein + 1/4 Gyr). The other breeds were Holstein (7/8 Holstein + 1/8 Gyr and 15/16 Holstein + 1/16 Gyr), Gyr, Jersey or crossbreed/Nelore.
Animal histories and blood collections were taken from at least 90% of the animals on each range. Owners in all regions of the state of Goiás were contacted, regardless of the type of ranch (dairy, beef or mixed). On each dairy farm, the history of daily milk production before and after the occurrence of T. vivax was investigated.
Parasitological diagnosis of T. vivax on ranches during the outbreak
The Woo method (Woo, 1970) was used to determine the presence of T. vivax in the blood samples. Approximately 4 mL of blood was collected from the caudal vein of each animal in a tube containing an anticoagulant (EDTA). Immediately after collection, the samples were homogenized and the blood was transferred to microhaematocrit tubes. Each tube was placed in a micro-centrifuge. After 5 min of centrifugation (13 000 RCF), the tube was examined under an optical microscope (magnification: 400×) for the study of T. vivax trypomastigotes.
Spatial distribution of T. vivax and risk factors
The state of Goiás is located in the midwestern region of Brazil and has an area of 340 106 km2. To facilitate the interpretation of the spatial distribution of the registered cases, the data were grouped by mesoregion. The division of mesoregions (central, eastern, northern, northwestern and southern) as well as the division of the municipalities in which T. vivax was detected in cattle followed the division established by the Brazilian Institute of Geography and Statistics (IBGE, 2019).
Possible risk factors for cattle to acquire T. vivax in the state of Goiás were determined using a questionnaire administered at each property visited. The questionnaire addressed the following: type of ranch (dairy, beef or mixed); animal category presenting problem (lactating cows, dry cows, heifers, calves, bulls, steers, calves); whether animals had been purchased in the previous 90 days and the place of purchase; whether abortions occurred in this period; whether sick animals presented difficulty moving; whether a reduction in milk production had recently occurred; whether oxytocin was administered to lactating cows and whether the same syringe and needle were shared among different animals; whether haematophagous flies (such as Tabanus, S. calcitrans or H. irritans) were present on the property; and whether artificial insemination was employed on the ranch.
Molecular identification of T. vivax and phylogenetic analysis
Blood samples were sequenced (18S rRNA gene) and the phylogenetic analysis was performed for 20 of the 24 ranches visited on which the parasitological diagnosis confirmed animals positive for T. vivax: Alexânia, Bonfinópolis 01, Bonfinópolis 02, Caldas Novas, Goianápolis, Inhumas, Ipameri 01, Ipameri 02, Ipameri 03, Ipameri 04, Ipameri 05, Ipameri 06, Itauçu 01, Itauçu 02, Morrinhos, Pontalina 01, Pontalina 02, Quirinópolis, Urutaí 01, Urutaí 02 and Urutaí 03. The positive control was Ipameri 01 registered in GenBank (accession code MK392089). The samples from the four remaining ranches (Campo Alegre de Goiás, Cromínia, Mairipotaba and Santa Bárbara de Goiás) that had animals infected with T. vivax were insufficient to perform the analyses.
The detection and molecular characterization of T. vivax were performed as described by Vieira et al. (2017). Genomic DNA was extracted from 200 μL of bovine blood using a commercial kit (Kasvi, Brazil) following the manufacturer's recommendations. Polymerase chain reaction (PCR) was performed using the primers 18STnF2 (5-CAACGATGACACCCATGAATTGGGGA-3) and 18STnR3 (5-TGCGCGACCAATAATTGCAATAC-3), which amplified a fragment of 659 bp of the 18S rRNA gene of T. vivax. The identity of the DNA sequences was determined by comparisons with sequences available in GenBank using BLASTn. A phylogenetic tree was constructed using the unweighted pair group method with arithmetic mean (UPGMA).
Statistical analysis
The data on the total number of animals diagnosed with T. vivax in the mesoregions and municipalities of the state of Goiás, Brazil, were used to calculate the prevalence. Subsequently, prevalence data were arranged in ascending order both for mesoregions and municipalities, setting the odds ratio (OR) equal to 1 for the lowest observed prevalence, then calculating the other ORs in relation to this. The Z test was used to determine the significance, considering a 95% significance level (P ⩽ 0.05).
Regression analysis was employed to determine associations between the prevalence [dichotomized by the median (zero for values below and one for values above the median)] of T. vivax and all the epidemiological variables described above. With these data, a simple binary logistic regression analysis was applied for all epidemiological variables and only those with a P value ⩽ 0.20 in the univariate analysis were selected for the multivariate logistic regression analysis. The strength of the associations between the dependent and independent variables was estimated using ORs derived from the logistic regression estimates. Variables with a P ⩽ 0.05 in the multivariate analysis were considered to be significantly associated with the outcome. A correlation analysis was also performed on the prevalence of T. vivax and epidemiological data collected during the visits. For such, Spearman's correlation coefficients were calculated. All statistical procedures were conducted using Epi Info, version 7.1.5.2.
Results
Prevalence of T. vivax in the state of Goiás during outbreaks
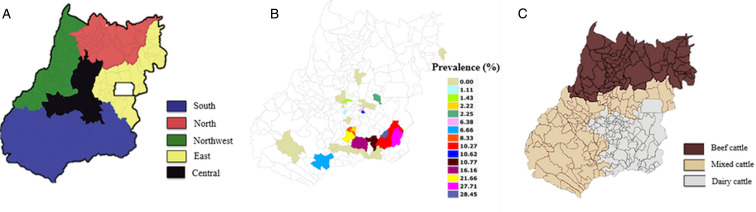
Trypanosoma vivax was found on 24 ranches distributed among 14 municipalities only in Girolando dairy cattle and only in lactating and/or dry cows. Among a total of 4049 blood samples analysed using the Woo method, 358 (8.84%; CI 95% 7.97–9.72) were positive for the acute form of trypanosomosis, as shown in Table 1 and Figs. 1A–C.
Table 1.
Association analysis between the mesoregions of the state of Goiás where Trypanosoma vivax was diagnosed in cattle
| Mesoregionsa | Total of animals | Mesoregion representativity (%) in relation to the total number of cattle evaluated | Protozoan | Prevalence (%) | Relative risk | Odds ratio | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | Value | 95% CI | Value | 95% CI | z statistic | Significance level | Value | 95% CI | z statistic | Significance level | |||||||||
| East | 589 | 14.55 | 2 | 587 | 0.34 | 0.13 | – | 0.81 | 1.00 | 1.00 | ||||||||||
| Central | 2032 | 50.19 | 194 | 1838 | 9.55 | 8.27 | – | 10.82 | 28.17 | 7.00 | – | 112.89 | 4.70 | <0.0001 | 30.98 | 7.67 | – | 125.14 | 4.82 | <0.0001 |
| South | 1428 | 35.27 | 162 | 1266 | 11.34 | 9.70 | – | 12.99 | 33.41 | 8.31 | – | 134.29 | 4.94 | <0.0001 | 37.56 | 30.12 | – | 46.82 | 32.22 | <0.0001 |
| – | 4049 | 100.0 | 358 | 3691 | 8.84 | 7.97 | – | 9.72 | – | – | – | – | – | – | – | – | – | – | ||
No positive animals were detected in North and Northwest mesoregions of the State.
Mesoregions with odds ratio and relative risk >1 (95% CI > 1) are more likely to contain cattle infected with Trypanosoma vivax
Fig. 1.
Mesoregions of Goiás State, Brazil (A). Spatial distribution of Trypanosoma vivax in Goiás State, Brazil between May 2015 and May 2017 (B). Distribution of cattle exploitation in Goiás State, Brazil (C). Adapted from Rocha et al. (2009).
Prevalence rates, ORs and RRs by mesoregion in the state of Goiás are displayed in Table 2 and Fig. 1B. No animals positive for T. vivax were found in 12 of the 26 municipalities of origin: Anápolis, Edealina, Buriti Alegre, Corumbaíba, Gameleira de Goiás, Goianésia, Goiatuba, Guapó, Itaberaí, Jataí, Mambaí and Porteirão. On four of the properties where T. vivax was not found (located in the municipalities of Mambaí, Buriti Alegre, Jataí and Quirinópolis), the blood analysis revealed high parasitism by Anaplasma marginale in cows, which was probably the cause of some deaths reported by the owners when answering the questionnaire.
Table 2.
Association analysis between the municipalities of the state of Goiás, regarding the prevalence of Trypanosoma vivax diagnosed in cattle
| Municipalsa | Mesoregion | Total of animals | Municipals representativity (%) in relation to the total number of cattle evaluated | Protozoan | Prevalence (%) | Relative risk | Odds ratio | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | Value | 95% CI | Value | 95% CI | z statistic | Significance level | Value | 95% CI | z statistic | Significance level | ||||||||||
| Anápolis | Central | 110 | 2.72 | 0 | 110 | 0.00 | |||||||||||||||
| Edealina | South | 25 | 0.62 | 0 | 25 | 0.00 | |||||||||||||||
| Buriti Alegre | Central | 27 | 0.67 | 0 | 27 | 0.00 | |||||||||||||||
| Corumbaíba | South | 25 | 0.62 | 0 | 25 | 0.00 | |||||||||||||||
| Gameleira de Goiás | South | 40 | 0.99 | 0 | 40 | 0.00 | |||||||||||||||
| Goianésia | Central | 10 | 0.25 | 0 | 10 | 0.00 | |||||||||||||||
| Goiatuba | South | 50 | 1.23 | 0 | 50 | 0.00 | |||||||||||||||
| Guapó | Central | 20 | 0.49 | 0 | 20 | 0.00 | |||||||||||||||
| Itaberaí | Central | 25 | 0.62 | 0 | 25 | 0.00 | |||||||||||||||
| Jataí | South | 120 | 2.96 | 0 | 120 | 0.00 | |||||||||||||||
| Mambaí | East | 500 | 12.35 | 0 | 500 | 0.00 | |||||||||||||||
| Porteirão | South | 20 | 0.49 | 0 | 20 | 0.00 | |||||||||||||||
| Santa Bárbara de Goiás | Central | 90 | 2.22 | 1 | 89 | 1.11 | 0.00 | – | 3.28 | 1.00 | 1.00 | ||||||||||
| Itauçu | Central | 140 | 3.46 | 2 | 138 | 1.43 | 0.00 | – | 3.39 | 1.29 | 0.12 | − | 13.97 | 0.21 | 0.8364 | 1.29 | 0.12 | – | 14.44 | 0.21 | 0.8364 |
| Cromínia | South | 45 | 1.11 | 1 | 44 | 2.22 | 0.00 | – | 6.53 | 2.00 | 0.13 | − | 31.24 | 0.49 | 0.6211 | 2.02 | 0.12 | – | 33.11 | 0.49 | 0.6213 |
| Alexânia | East | 89 | 2.20 | 2 | 87 | 2.25 | 0.00 | – | 5.33 | 2.02 | 0.19 | − | 21.91 | 0.58 | 0.5623 | 2.05 | 0.18 | – | 22.98 | 0.58 | 0.5618 |
| Goianápolis | Central | 47 | 1.16 | 3 | 44 | 6.38 | 0.00 | – | 13.37 | 5.74 | 0.61 | − | 53.72 | 1.53 | 0.1253 | 6.07 | 0.61 | – | 60.03 | 1.54 | 0.1231 |
| Mairipotaba | South | 12 | 0.30 | 1 | 11 | 8.33 | 0.00 | – | 23.97 | 7.50 | 0.50 | − | 112.23 | 1.46 | 0.1444 | 8.09 | 0.47 | – | 138.73 | 1.44 | 0.1493 |
| Quirinópolis | South | 20 | 0.49 | 2 | 18 | 10.00 | 0.00 | – | 23.15 | 9.00 | 0.86 | − | 94.47 | 1.83 | 0.0670 | 9.89 | 0.85 | – | 114.98 | 1.83 | 0.0672 |
| Ipameri | South | 584 | 14.42 | 60 | 524 | 10.27 | 7.81 | – | 12.74 | 9.25 | 1.30 | − | 65.89 | 2.22 | 0.0264 | 10.19 | 1.39 | – | 74.48 | 2.29 | 0.0222 |
| Bonfinópolis | Central | 1440 | 35.56 | 153 | 1287 | 10.63 | 9.03 | – | 12.22 | 9.56 | 1.35 | − | 67.54 | 2.26 | 0.0236 | 10.58 | 1.46 | – | 76.49 | 2.34 | 0.0194 |
| Caldas Novas | South | 65 | 1.61 | 7 | 58 | 10.77 | 3.23 | 18.31 | 9.69 | 1.22 | − | 76.88 | 2.15 | 0.0316 | 10.74 | 1.29 | – | 89.60 | 2.19 | 0.0283 | |
| Morrinhos | South | 99 | 2.45 | 16 | 83 | 16.16 | 8.91 | – | 23.41 | 14.55 | 1.97 | − | 107.48 | 2.62 | 0.0087 | 17.16 | 2.23 | – | 132.25 | 2.73 | 0.0064 |
| Pontalina | South | 240 | 5.93 | 52 | 188 | 21.67 | 16.45 | – | 26.88 | 19.50 | 2.74 | − | 138.97 | 2.96 | 0.0030 | 24.62 | 3.35 | – | 180.95 | 3.15 | 0.0016 |
| Campo Alegre de Goiás | South | 83 | 2.05 | 23 | 60 | 27.71 | 18.08 | – | 37.34 | 24.94 | 3.44 | − | 180.60 | 3.18 | 0.0015 | 34.12 | 4.49 | – | 259.44 | 3.41 | 0.0006 |
| Urutaí | Central | 123 | 3.04 | 35 | 88 | 28.46 | 20.48 | – | 36.43 | 25.61 | 3.57 | − | 183.48 | 3.23 | 0.0012 | 35.40 | 4.75 | – | 264.06 | 3.48 | 0.0005 |
| – | – | 4049 | 100.00 | 358 | 3691 | 8.84 | 7.97 | – | 9.72 | – | – | – | – | – | – | – | – | – | – | ||
Municipals with odds ratio and relative risk >1 (95% CI > 1) are more likely to contain cattle infected with Trypanosoma vivax.
Risk factors associated with epidemiological variables
Among the epidemiological variables submitted to logistic regression analysis, the type of animal utilization (beef, dairy or mixed), animal category (lactating cows and others), purchase of animals in the previous 90 days, a reduction in milk production and the use of oxytocin in lactating cows sharing the same syringe and needle were significantly associated (P ⩾ 0.05) with the occurrence of T. vivax in cattle in the state of Goiás, Brazil (Table 3).
Table 3.
Association between the prevalence of Trypanosoma vivax diagnosed in the state of Goiás with some epidemiological variables using logistic regression analysis
| Epidemiologicala variable | Odds ratio | 95% CI | Significance level | ||
|---|---|---|---|---|---|
| Type of utilization (dairy, beef or mixed) | 1.3175 | 1.1309 | – | 1.5349 | 0.0004 |
| Animal category | 1.4096 | 1.2505 | 1.5889 | < 0.00001 | |
| Animal purchased in the last 90 days | 2.6009 | 2.0057 | – | 3.3726 | < 0.00001 |
| Use of exogenous oxytocin and sharing of syringe and needle | 18.4205 | 12.3217 | – | 27.5379 | < 0.00001 |
| Sudden impact on daily milk production | 1.0002 | 1.0002 | – | 1.0003 | < 0.00001 |
Epidemiological variable with odds ratio >1 (95% CI > 1) is more likely to contain cattle infected with Trypanosoma vivax.
Specifically, significant positive correlations (P ⩽ 0.05) were found between the prevalence of T. vivax and animal category (ρ = 0.78), type of cattle (ρ = 0.68), purchase of animals in the previous 90 days (ρ = 0.38), place of purchase of these animals (ρ = 0.87) and use of oxytocin in cows during milking sharing the same syringe and needle (ρ = 0.69). The purchase of animals in the previous 90 days also had a significant positive correlation (P ⩽ 0.05) with the occurrence of abortions (ρ = 0.69), locomotion difficulty of the animals, a sudden reduction in daily milk production (ρ = 0.54) and the place of purchase of these animals (ρ = 0.41). Significant positive correlations (P ⩽ 0.05) were also found between the place of purchase of the animals and the type of cattle (ρ = 0.40) and the use of oxytocin in the cows during milking sharing the same syringe and needle (ρ = 0.38).
The mean daily production of milk before the outbreaks (1595 L ± 1201.9) on the 24 properties where T. vivax was diagnosed was higher (P ⩽ 0.05) than the mean number of litres produced daily (963.0 ± 686.2) on these same properties after the outbreaks caused by the infection of this protozoan. In other words, the occurrence of T. vivax (±90 days) led to a 39.62% reduction in average daily milk production.
Molecular identification of T. vivax and phylogenetic analysis
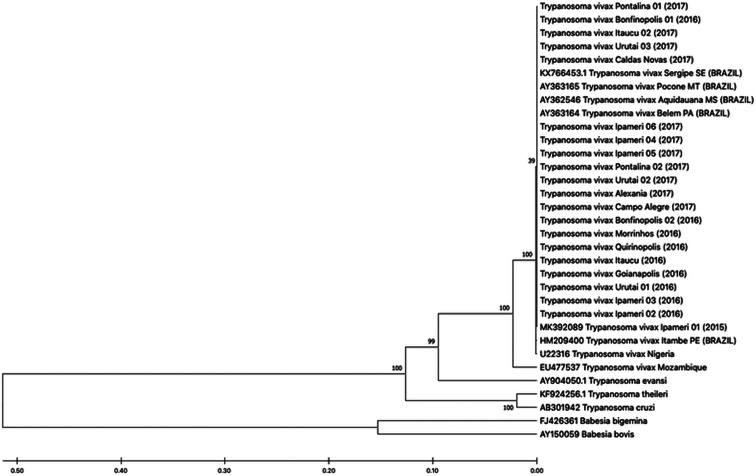
In the molecular diagnosis (PCR), 100% (20/20) of the samples tested positive for T. vivax. The sequences from the NCBI GenBank used to perform the phylogenetic analyses were Belém (AY363164), Poconé (AY363165), Aquidauana (AY362546), Ipameri (MK392089), Itambé (HM209400), São Miguel Aleixo (KX766453.1), Nigeria (U22316), Mozambique (EU477537), Trypanosoma evansi (AY904050.1), Trypanosoma theileri (KF924256.1), Trypanosoma cruzi (AB301942), Babesia bigemina (FJ426361) and Babesia bovis (AY150059). In the alignment of the DNA sequences, all T. vivax in this study were genetically homologous to T. vivax found in different states of Brazil (Mato Grosso, Mato Grosso do Sul, Pará, Pernambuco and Sergipe) and Nigeria (west Africa), as demonstrated by comparisons to sequences in the NCBI GenBank database. The homology search of the sequenced amplicons revealed 100% identity among the sequences of the 18S rRNA gene. However, we noticed a difference in comparison to T. vivax from Mozambique (east Africa) (Fig. 2).
Fig. 2.
Phylogenetic tree based on 18SrRNA genes sequences of Trypanosoma vivax isolates (Brazil, Nigeria and Mozambique), Trypanosoma evansi, Trypanosoma theileri, Trypanosoma cruzi, Babesia bigemina and Babesia bovis. Sequences were compared using the unweighted pair group method with arithmetic mean (UPGMA). The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) is shown next to the branches. The scale bar represents the number of mutations per sequence position.
Discussion
This work reports novel findings regarding epidemiological and genetic aspects of trypanosomosis in Brazil. Significant associations were found between the prevalence of T. vivax and the type of cattle, the purchase of animals and use of oxytocin with the same syringe and needle in cows during milking. These aspects explain the rapid dissemination of this protozoan parasite among dairy cattle in this study.
The prevalence of T. vivax in cattle is causally related to the sensitivity of the diagnostic method employed. In a comparative study of indirect immunofluorescence, conventional PCR (cPCR) and the Woo method, cPCR proved the most sensitive for detecting low parasitaemia (Alves et al., 2017; Rabelo et al., 2017; Bastos et al., 2020). However, the Woo method can be used to identify animals during the acute phase of infection in outbreaks of trypanosomosis, which justifies the use of this method in the present investigation, the aim of which was to diagnose acute cases of trypanosomosis in cattle.
Over the past 16 years, T. vivax has spread rapidly across Brazil (Linhares et al., 2006; Batista et al., 2007; Carvalho et al., 2008; Guerra et al., 2008; Silva et al., 2009; Cadioli et al., 2012; Pimentel et al., 2012; Andrade Neto e André, 2015; Costa et al., 2016; Bastos et al., 2017; Vieira et al., 2017; Lopes et al., 2018). In 11 of the publications cited, outbreaks occurred in Girolando dairy cattle; 10 reported the introduction of new animals to the herd and seven reported the use of oxytocin and/or vaccines as the predisposing factor for the occurrence of trypanosomosis in the respective herds. The sale of these animals occurs collectively at auctions, which directly contributes to the dissemination of this protozoan to other properties. The use of intravenous oxytocin in lactating cows is a common management practice to increase the milking speed and reduce the time spent milking on properties with Girolando dairy cows (Araújo et al., 2012). This practice is performed with the same needle and syringe on several animals, which contributes to the spread of diseases in the herd. Thus, in view of the results obtained in the present investigation and previous studies, we can state that the acquisition of new animals with T. vivax and the administration of exogenous oxytocin to cows during milking using the same syringe and needle are the main causes of the dissemination of trypanosomosis in the state of Goiás and other regions of Brazil.
The 39.62% reduction in average daily milk production found on properties with the presence of T. vivax may be explained by the persistent fever that the acute phase of this disease causes in cattle with high parasitaemia (⩾2 × 106). It is well known that sudden, persistent fever results in anorexia in animals (Cavalcante, 2000; Peixoto et al., 2011). Moreover, other researchers describe a positive correlation with hyperthermia in cattle during the acute phase of trypanosomosis, which lends support to the inference offered here (Schenk et al., 2001; Desquesnes, 2004; Almeida et al., 2010; Dagnachew and Bezie, 2015).
The presence of tabanid flies, S. calcitrans or H. irritans, was not correlated (P > 0.05) with infection on the 24 properties in which T. vivax outbreaks were diagnosed. However, researchers have reported that animal–animal transmission in other regions of the country could be related to the presence of these mechanical vectors (Otte and Abuabara, 1991; Silva et al., 1996; Batista et al., 2012; Cadioli et al., 2012; Batista et al., 2018). The epidemiological data demonstrate the importance of restricting the practice of collective sales events (auctions) as well as eliminating the use of exogenous oxytocin in animals during the milking process to avoid serious problems for cattle breeders in Brazil. Therefore, further studies evaluating the dissemination capacity of T. vivax transmission by haematophagous flies and studies evaluating the propagation capacity of iatrogenic pathways of T. vivax are needed.
The phylogenetical analysis proved that the T. vivax found in Goiás, Brazil, was homologous to others from the same country and west Africa, but different from T. vivax from east Africa. These results are in agreement with data in the literature stating that T. vivax in Brazil was originally from Africa (probably west Africa) (Osório et al., 2008; Cortez et al., 2006; Rodrigues et al., 2008; Pimentel et al., 2012; Vieira et al., 2017).
Conclusion
The prevalence of trypanosomosis in the state of Goiás, Brazil, during the acute phase of the disease was 8.84%. The main risk factors for the dissemination of T. vivax in the herds were the acquisition of new animals infected with this protozoan and the administration of oxytocin in cows using the same syringe and needle among several animals. In contrast, the presence of tabanid flies, S. calcitrans and H. irritans, was not a risk factor for the occurrence of this haemoparasite. The T. vivax identified in the present study were genetically homologous to each other as well as others found in Brazil and west Africa.
Financial support
This work was supported by Fundo para o Desenvolvimento da Agropecuária em Goiás (FUNDEPEC-GOIÁS) (agreement signed on 5 May 2016) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – financial code 001.
Ethical standards
This study received approval from the Animal Use Ethics Committee of the Federal University of Goiás, Brazil (certificate number: 032/15) and was conducted in compliance with the ethical principles governing animal experimentation of the Brazilian National Animal Experimentation Control Council (CONCEA).
References
- Almeida KS, Freitas FLC, Tebaldi JH, Alessi AC, Machado RZ and Nascimento AA (2010) Alterações clínicas, histopatológicas e enzimáticas em ovinos infectados experimentalmente por Trypanosoma vivax. Ciência Animal Brasileira 11, 669–676. [Google Scholar]
- Andrade Neto AQ, Afonso JAB, Mendonça CL, Souto RJC, André MR and Machado RZ (2015) Surtos de tripanossomiase em bovinos leiteiros no agreste dos estados de Pernambuco e Alagoas. Biológico 77, 143. [Google Scholar]
- Araújo WAGD, Carvalho CGV, Marcondes MI, do Sacramento AJR and Paulino PVR (2012) Ocitocina exógena e a presença do bezerro sobre a produção e qualidade do leite de vacas mestiças. Brazilian Journal of Veterinary Research and Animal Science 49, 465–470. [Google Scholar]
- Bastos TSA, Faria AM, Madrid DMDC, Bessa LCD, Linhares GFC, Fidelis Junior OL, Sampaio PH, Cruz BC, Cruvinel LB, Nicaretta JE, Machado RZ, Costa AJ and Lopes WDZ (2017) First outbreak and subsequent cases of Trypanosoma vivax in the state of Goiás, Brazil. Revista Brasileira de Parasitologia Veterinária 23, 366–371. [DOI] [PubMed] [Google Scholar]
- Bastos TSA, Faria AM, Cavalcante ASA, Madrid DMC, Zapa DMB, Nicaretta JE, Cruvinel LB, Heller LM, Couto LMF, Rodrigues DC, Ferreira LL, Soares VE, Cadioli FA and Lopes WDZ (2020) Infection capacity of Trypanosoma vivax experimentally inoculated through different routes in bovines with latent Anaplasma marginale. Experimental Parasitology 211, 1–8 [DOI] [PubMed] [Google Scholar]
- Batista JS, Riet-Correa F, Teixeira MMG, Madruga CR, Simões SDV and Maia TF (2007) Trypanosomosis by Trypanosoma vivax in cattle in the Brazilian semiarid: description of an outbreak and lesions in the nervous system. Veterinary Parasitology 143, 174–181. [DOI] [PubMed] [Google Scholar]
- Batista JS, Rodrigues CMF, Olinda RG, Silva TMF, Vale RG, Câmara ACL, Rebouças RES, Bezerra FSB, García HÁ and Teixeira MMG (2012) Highly debilitating natural Trypanosoma vivax infections in Brazilian calves: epidemiology, pathology, and probable transplacental transmission. Parasitology Research 110, 73–80. [DOI] [PubMed] [Google Scholar]
- Batista JS, Moura GHF, Lopes FC, Paiva KARD, Araújo Júnior HND, Góis RCDS, Costa KMFM, Coelho WAC and Freitas CIA (2018) Risk factors for trypanosomosis by Trypanosoma vivax in cattle raised in Rio Grande do Norte state. Arquivos do Instituto Biológico 85, e0232016. [Google Scholar]
- Cadioli FA, Barnabé P, Machado RZ, Teixeira MCA, André MR, Sampaio PH, Fidélis Junior OL, Teixeira MMG and Marques LC (2012) First report of Trypanosoma vivax outbreak in dairy cattle in São Paulo state, Brazil. Revista Brasileira de Parasitologia Veterinária 21, 118–124. [DOI] [PubMed] [Google Scholar]
- Carvalho AU, Abrão DC, Facury Filho EJ, Paes PRO and Ribeiro MFB (2008) Occurrence of Trypanosoma vivax in Minas Gerais state, Brazil. Arquivo Brasileiro de Medicina Veterinária e Zootecnia 60, 769–771. [Google Scholar]
- Cavalcante FA (2000) Rinotraqueíte infecciosa bovina (nariz vermelho), diagnóstico e controle. Embrapa Acre-Séries anteriores (INFOTECA-E).
- Cortez AP, Ventura RM, Rodrigues AC, Batista JS, Paiva F, Añez N, Machado RZ, Gibson WC and Teixeira MMG (2006) The taxonomic and phylogenetic relationships of Trypanosoma vivax from South America and Africa. Teixeira MMG 133, 159–169 [DOI] [PubMed] [Google Scholar]
- Costa RVC, Abreu APM, Machado MN, Thomé SMG, Massard CL, Santos HA and Brito MF (2016) Tripanossomíase em bovinos no estado do Rio de Janeiro. Pesquisa Veterinária Brasileira 36, 161–163. [Google Scholar]
- Cuglovici DA, Bartholomeu DC, Reis-Cunha JL, Carvalho AU and Ribeiro MFB (2010) Epidemiologic aspects of an outbreak of Trypanosoma vivax in a dairy cattle herd in Minas Gerais state, Brazil. Veterinary Parasitology 169, 320–326. [DOI] [PubMed] [Google Scholar]
- Dagnachew S and Bezie M (2015) Review on Trypanosoma vivax. African Journal of Basic and Applied Science 7, 41–64. [Google Scholar]
- Desquesnes M (2004) Livestock Trypanosomoses and Their Vectors in Latin America, vol. 8. France: OIE – World Organisation for Animal Health, 174p. [Google Scholar]
- Guerra RDMSN, Júnior F, Batista A, Santos HP, Abreu-Silva AL and Santos ACGD (2008) Biometry of Trypanosoma vivax found in a calf in the state of Maranhão, Brazil. Ciência Rural 38, 833–835. [Google Scholar]
- IBGE – Instituto Brasileiro de Geografia e Estati´stica (2019) Censo agropecuário de 2008. Available at http://www.cidades.ibge.gov.br (Accessed 06 January 2019).
- Linhares GFC, Dias Filho FC, Fernandes PR and Duarte SC (2006) Tripanossomíase em bovinos no município de Formoso do Araguaia, Tocantins (relato de caso). Ciência Animal Brasileira 7, 455–460. [Google Scholar]
- Lopes STP, Prado BDS, Martins GHC, Esmeraldo H, Beserra A, de Sousa Filho MAC, Evangelista LSDM and Souza JATD (2018) Trypanosoma vivax em bovino leiteiro. Acta Scientiae Veterinariae 46, 287. [Google Scholar]
- Osório ALAR, Madruga CR, Desquesnes M, Soares CO, Ribeiro LRR and Costa SCGD (2008) Trypanosoma (Duttonella) vivax: its biology, epidemiology, pathogenesis, and introduction in the New World – a review. Memórias do Instituto Oswaldo Cruz 103, 1–13. [DOI] [PubMed] [Google Scholar]
- Otte MJ and Abuabara JY (1991) Transmission of South American Trypanosoma vivax by the neotropical horsefly Tabanus nebulosus. Acta Tropica 49, 73–76. [DOI] [PubMed] [Google Scholar]
- Paiva F, Lemos RAAD, Nakasato L, Mori AE, Brum KB and Bernardo KC (2000) Trypanosoma vivax em bovinos no Pantanal do Estado do Mato Grosso do Sul, Brasil: I.-Acompanhamento clínico, laboratorial e anatomopatológico de rebanhos infectados. Revista Brasileira de Parasitologia Veterinária 9, 135–141. [Google Scholar]
- Peixoto PV, Cunha BM, França TN, Bezerra Junior PS, Brust LAC, Terra TMF and Armién AG (2011) Hereditary encephalopathy of cattle in Espírito Santo state, Brazil. Pesquisa Veterinária Brasileira 31, 723–730. [Google Scholar]
- Pimentel DS, Ramos CADN, Ramos RADN, de Araújo FR, Borba ML, Faustino MADG and Alves LC (2012) First report and molecular characterization of Trypanosoma vivax in cattle from state of Pernambuco, Brazil. Veterinary parasitology 185, 286–289. [DOI] [PubMed] [Google Scholar]
- Rabelo AML, Teodoro PE, Peixoto LA, Silva LA and Laviola BG (2017) Comparison of three methods for diagnosis of Trypanosoma (Duttonella) vivax in cattle. Genetics and Molecular Research 16, 1–7. [Google Scholar]
- Rocha WV, Gonc¸alves VSP, Coelho CGFL, Brito WME, Dias R, Delphino M, Ferreira F, Amaku M, Ferreira Neto JS, Figueiredo VC, Lo^bo JR and Brito LAB (2009) Epidemiological status of bovine brucellosis in the State of Goiás, Brazil. Arquivo Brasileiro de Medicina Veterinária e Zootecnia 61, 27–34. [Google Scholar]
- Rodrigues AC, Neves L, Garcia HA, Viola LB, Marcili A, Da Silva FM, Sigauque I, Batista JS, Paiva F and Teixeira MMG (2008) Phylogenetic analysis of Trypanosoma vivax supports the separation of South American/West African from East African isolates and a new T. vivax-like genotype infecting a nyala antelope from Mozambique. Parasitology 135, 1317–1328 [DOI] [PubMed] [Google Scholar]
- Schenk MAM, Mendonça CL, Madruga CR, Kohayagawa A and Araújo FR (2001) Avaliação clínico-laboratorial de bovinos Nelore infectados experimentalmente com Trypanosoma vivax. Pesquisa Veterinária Brasileira 21, 157–161. [Google Scholar]
- Shaw JJ and Lainson R (1972) Trypanosoma vivax in Brazil. Annals of Tropical Medicine and Parasitology 66, 25–32. [DOI] [PubMed] [Google Scholar]
- Silva RAMS, Silva JAD, Schneider RC, Freitas JD, Mesquita D, Mesquita T, Laura R, Dávila AMR and Pereira MEB (1996) Outbreak of trypanosomosis due to Trypanosoma vivax (Ziemann, 1905) in bovines of the Pantanal, Brazil. Memórias do Instituto Oswaldo Cruz 91, 561–562. [DOI] [PubMed] [Google Scholar]
- Silva ASD, Costa MM, Polenz MF, Polenz CH, Teixeira MMG, Lopes STDA and Monteiro SG (2009) Primeiro registro de Trypanosoma vivax Em bovinos no Estado do Rio Grande do Sul, Brasil. Ciência Rural 39, 2550–2554. [Google Scholar]
- Ventura RM, Paiva F, Silva RAMS, Takeda GF, Buck GA and Teixeira MMG (2001) Trypanosoma vivax: characterization of the spliced-leader gene of a Brazilian stock and species-specific detection by PCR amplification of an intergenic spacer sequence. Experimental Parasitology 99, 37–48. [DOI] [PubMed] [Google Scholar]
- Vieira OLE, Macedo LOD, Santos MAB, Silva JABA, Mendonça CLD, Faustino MADG, Ramos CAN, Alves LC, Ramos RAN and Carvalho GA (2017) Detection and molecular characterization of Trypanosoma (Duttonella) vivax in dairy cattle in the state of Sergipe, northeastern Brazil. Revista Brasileira de Parasitologia Veterinária 24, 516–520. [DOI] [PubMed] [Google Scholar]
- Woo PTK (1970) The haematocrit centrifuge technique for the diagnosis of African trypanosomosis. Acta Tropica 27, 384–386. [PubMed] [Google Scholar]