Abstract
In areas endemic for Leishmania infantum, an asymptomatic infection may be an indicator of the extent of transmission. The main goal of this study was to evaluate the applicability of measuring circulating immunological biomarkers as an alternative strategy to characterize and monitor L. infantum asymptomatic infections in combination with serological methods. To this end, 179 children from a region endemic for visceral leishmaniasis (VL), aged 1–10 years old, selected from a cross-sectional study, were identified as asymptomatic (n = 81) or uninfected (n = 98) by qPCR and/or serological tests (ELISA using L. infantum soluble antigen and rK39), and, together with serum samples of children diagnosed with VL (n = 43), were subjected to avidity tests and cytokine levels measurement. Avidity rates (AR) ranging from 41 to 70% were found in 29 children (66%) from the asymptomatic group. On the other hand, high AR (above 70%) were observed in 27 children (64%) from the VL group. Logistic Regression and Classification and Regression Tree (CART) analyses demonstrated that lower AR and IFN-γ production associated with higher IL-17A levels were hallmarks in asymptomatic L. infantum infections. Therefore, this study proposes an association of immunological biomarkers that can be used as a complementary strategy for the characterization and monitoring of asymptomatic VL infections in children living in endemic areas.
Key words: Asymptomatic L. infantum infection, immunological biomarkers, visceral leishmaniasis
Introduction
Visceral leishmaniasis (VL) is a neglected tropical disease that accounts for approximately 200 000–400 000 new cases worldwide (Alvar et al., 2012; WHO, 2019). In the Americas, the etiologic agent of VL is Leishmania (Leishmania) infantum. The disease is present in 12 countries, with 90% of the cases being reported in Brazil (4200–6300 cases per year), an incidence rate of 2.0/100 000 inhabitants, and a fatality rate of around 7% (Romero and Boelaert, 2010). Although VL is potentially fatal if not promptly diagnosed and treated, the L. infantum infection in humans does not always lead to clinical illness. Different studies conducted in endemic areas in Brazil have shown that individuals harbouring asymptomatic infections are frequently found and seem to be more numerous than clinical cases (Badaro et al., 1986; Evans et al., 1992; Jerônimo et al., 2000; Caldas et al., 2001; Braz et al., 2002; Costa et al., 2002; Moreno et al., 2006; de Gouvêa Viana et al., 2008; Moreno et al., 2009). Ratios of eight to 18 incident asymptomatic infections to one incident clinical case have been described (Badaro et al., 1986; Costa et al., 1999; Costa et al., 2002; Romero and Boelaert, 2010).
It should be emphasized, however, that the relevance of asymptomatic infection in parasite transmission and disease outcome is largely unknown. The identification, management and understanding of the biological and epidemiological implications of asymptomatic infection should be used for planning and evaluating VL control programme strategies. Nevertheless, the laboratory methods currently employed, such as serological and molecular tools, are still unsatisfactory. ELISA with L. infantum soluble antigen or recombinant K39 and polymerase chain reaction (PCR) tests have been performed in individuals who are otherwise in a healthy condition (Burns et al., 1993; Badaró et al., 1996; Moreno et al., 2006; de Gouvêa Viana et al., 2008), but present considerable discordance of results (Badaro et al., 1986; Evans et al., 1992; Badaró et al., 1996; Costa et al., 1999; Jerônimo et al., 2000; Caldas et al., 2001; Braz et al., 2002; Costa et al., 2002; Moreno et al., 2006; de Gouvêa Viana et al., 2008; Moreno et al., 2009; dos Santos Marques et al., 2012).
Thus, it becomes imperative to search for new biomarkers to assist in the characterization and monitoring of asymptomatic individuals. Different studies have shown basal immunological biomarker levels in asymptomatic VL infections (Peruhype-Magalhães et al., 2006; Khoshdel et al., 2009; Costa et al., 2012). On the other hand, studies using antigen-stimulated cells have identified and suggested the use of biomarkers, such as CXCL-10 and CXCL9 chemokines (Ibarra-Meneses et al., 2017; Porcino et al., 2019) and IL-12, IL-2, IFN-γ, IL-17A, IL-22, IL-4, and IL-10 cytokines (Peruhype-Magalhães et al., 2005; Pitta et al., 2009; Ibarra-Meneses et al., 2017), for the detection of L. infantum-infected individuals. These findings corroborate the hypothesis that cell immunity is present and usually persists for several years. The presence of memory cells (CD4+CD45RO+) and the production of different biomarkers after contact with the parasite (Rodrigues-Neto et al., 2018) are useful to characterize and monitor L. infantum-infected individuals. In this context, the main goal of this study was to evaluate the applicability of measuring circulating immunological biomarkers as an alternative strategy for characterizing and monitoring L. infantum asymptomatic infection in combination with serological methods.
Material and methods
Study design
This is a cohort study conducted in 2013 with 179 children aged 1–10 years old (mean age: 5 years old) selected from a cross-sectional study carried out in 2012 to evaluate the prevalence of asymptomatic L. infantum infection in an endemic area of Belo Horizonte, the capital of the state of Minas Gerais, Brazil. Among those, 141 children were diagnosed positive in at least one serological test (ELISA using recombinant antigen K39 and/or soluble L. infantum antigen performed in peripheral blood samples absorbed on filter paper), while 38 children were diagnosed negative in the two tests (da Rocha et al., 2018).
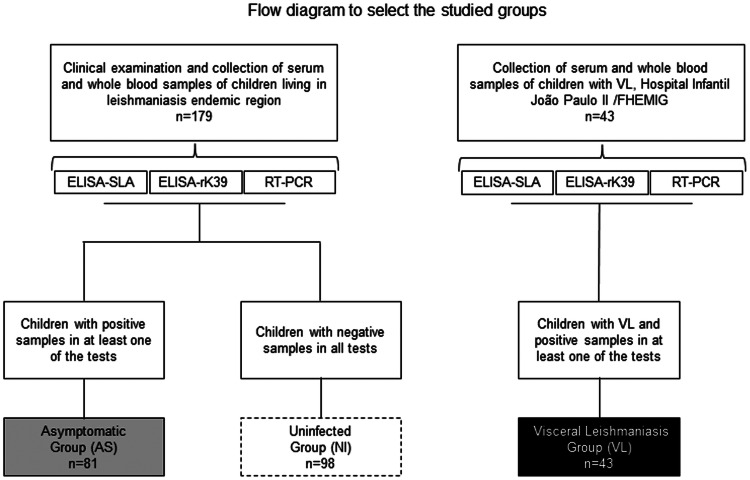
In the present study, the children were examined by medical specialists in health centres to detect signs and symptoms of VL, and peripheral blood samples were collected to obtain the serum. The children were subdivided into the following groups (Fig. 1):
Fig. 1.
Flow diagram to select the studied groups.
Asymptomatic group (AS)
This group was formed by 81 children (32 girls and 49 boys). These were diagnosed positive in at least one of the serological or molecular tests (ELISA using recombinant antigen K39 and/or soluble L. infantum antigen and/or qPCR) but showed no signs and symptoms of VL.
Uninfected group (NI)
This group was comprised of 98 children (51 girls and 47 boys), who were negative in both serological tests (ELISA using recombinant antigen K39 and soluble L. infantum antigen) and for the SSU-rDNA gene of Leishmania spp. by qPCR.
Additionally, 43 children (21 girls and 22 boys), aged 3 months to 10 years old (mean age: 3 years old), from the Hospital Infantil João Paulo II (HIJPII) – FHEMIG (Fundação Hospitalar do Estado de Minas Gerais) were included to comprise the VL group. These children presented clinical history suggestive of VL associated with positive parasitological tests (direct search for amastigotes of Leishmania spp.) and/or serological diagnosis (indirect immunofluorescence antibody test-IFAT and/or immunochromatographic rK39 antigen test). Children in this group were also tested by ELISA using recombinant antigen K39 and soluble L. infantum antigen and q-PCR, similar to the groups NI and AS (Fig. 1).
Enzyme-linked immunosorbent assays (ELISA-SLA and ELISA-rK39)
Soluble Leishmania antigen (SLA) was prepared using promastigotes of L. infantum (MHOM/BR/2002/LPC-RPV) as described by Ho et al. (1983), with minor modifications. Promastigotes were cultivated in NNN/LIT medium for 7 days and 9 × 107 parasites per mL were sonicated six times for 20 s on ice. Next, the suspension was centrifuged at 10 000 × g for 30 min at 4°C and the supernatant was stored in aliquots at −70°C until use. Protein concentration was determined using Bio-Rad Protein Assay (Bio-Rad Laboratories, Inc, Hercules, CA, USA).
NUNC Maxisorp polystyrene microplates (NUNC MaxiSorp™ Thermo Fisher Scientific, Rochester, NY, US catalogue number: 44-2404-21) were coated with 50 μL per well of soluble antigen of L. infantum diluted at 3 μg mL−1 in 0.1 m carbonate-bicarbonate buffer, pH 9.6 for approximately 18 h at 2–8°C. The microplates were washed five times with 0.15 m phosphate-buffered saline, pH 7.2 containing 0.05% Tween 20 (PBS-T). Next, 200 μL of 5% non-fat milk diluted in PBS-T (PBS-T-milk 5%) were added to each well and incubated for 2 h at 37°C, to saturate the free sites of the microplates. The microplates were washed again five times with PBS-T and 50 μl of serum diluted 1:1000 were deposited into the wells and incubated for 1 h at 37°C. Following another set of washes, 50 μL of conjugate anti-human IgG linked to peroxidase (Sigma Chemical Co., St Louis, MO, US catalogue number: A8419) diluted 1: 10 000 in PBS-T-milk 1% were added to each well and incubated for 1 h at 37°C. The microplates were washed again and 50 μL per well of chromogenic solution (H2O2 + TMB – Sigma Chemical Co., US catalogue number: T0440) were added. The assay was incubated for 5 min at room temperature in the dark and the enzymatic reaction was stopped with 50 μL per well of 1N sulfuric acid solution. The absorbance readings were performed at 450 nm in a microplate reader (Model 550, Bio-Rad Laboratories, Tokyo, Japan).
To perform the ELISA-rK39 (ELISA using recombinant antigen K39, kindly donated by Steven G. Reed, Infectious Disease Research Institute, Seattle, WA, USA), we used NUNC Maxisorp microplates (NUNC MaxiSorp™ Thermo Fisher Scientific, US catalogue number: 44-2404-21) coated with 50 μL per well of rK39 antigen (1 μg mL−1). Serum samples diluted 1:100 and anti-human IgG linked to peroxidase conjugate (Sigma Chemical Co., US catalogue number: A8419) diluted 1:50 000 in PBS-T-milk 1% were used following the same procedures mentioned above for the ELISA-SLA. Each serum was assayed in duplicate and the mean absorbance reading was assessed as the final result. Negative samples (n = 15) collected from staff team members resident in non-endemic areas of Belo Horizonte and a positive (n = 1) control serum were included in each run. The assay was considered validated when the variation of the absorbance reading values for the positive control serum was lower than 20%. Samples that showed differences greater than 20% between the absorbance reading values for the duplicates were retested. For each sample, the reactivity index (RI) was calculated by dividing the mean absorbance reading by the cut-off, established as the mean absorbance from negative control sera plus three standard deviations. Samples that presented RI⩾1.1 were considered positive and samples that presented RI<1.1 were considered negative.
Real-time polymerase chain reaction (qPCR)
DNA was extracted from blood samples using the Maxwell® 16 Lev Blood DNA kit (Promega, Madison, WI, US catalogue number: AS1290) and the Maxwell® 16 Instrument (Promega), according to the manufacturer's instructions. DNA concentration and purity were measured at 260 nm and 260/280 nm in a spectrophotometer NanoDrop™ ND-1000 (Thermo Fisher Scientific, Wilmington, DE, USA).
The ribosomal RNA small subunit gene (SSU rRNA) was chosen as the target of amplification. The forward LEIS.U1 (5′-AAGTGCTTTCCCATCGCAACT-3′) and the reverse LEIS.L1 (5′-GACGCACTAAACCCCTCCAA-3′) primers were used to amplify a 67-base pair (bp) fragment of the SSU rRNA gene of Leishmania spp. (Van Eys et al., 1992). Amplification was detected using a probe LEIS.P1 (FAM 5′-CGGTTCGGTGTGTGGCGCC-3′TAMRA) (Wortmann et al., 2001). The reactions were performed in duplicate in a final volume of 20 μL containing 10 μL of TaqMan MasterMix (Thermo Fisher Scientific, Foster, CA, US catalogue number: 4304437), 0.3 μm LEIS.L1 and LEIS.U1 primers, 0.25 μm LEIS.P1 probe, and 3 μL of extracted DNA diluted 1:10 in ultrapure water. Amplification was performed as follows: 50°C for 2 min for activation of the uracil DNA glycosylase, and 95°C for 10 min followed by 40 cycles of 95°C for 15 s and 60°C for 1 min in a SepOnePlusTM Real-Time PCR System (Thermo Fisher Scientific). Threshold of detection and baseline values were automatically determined using the StepOne software, v2.1 (Thermo Fisher Scientific).
As a control for DNA extraction, amplification and quality, and for the qPCR assays, a sequence of the β-actin human gene (ACTB) was amplified in parallel. The primers Aco1: 5′-CCA TCT ACG AGG GGT ATG-3′ and Aco2: 3′-GGT GAG GAT CTT CAT GAG GTA-5′ (Musso et al., 1996), were used to amplify a 120-bp fragment of the gene. The reaction was performed in duplicate in a final volume of 20 μL containing 10 μL of SYBR Green MasterMix (Thermo Fisher Scientific, US catalogue number: 4304437), 0.15 μm Aco1 and Aco2 primers, and 3 μL of extracted DNA diluted 1:10 in ultrapure water. The universal cycling parameters were used and the melting analysis was conducted based on the parameters of the StepOnePlus Thermal Cycler; the melting curve was analysed using the StepOne software, v2.1 (Thermo Fisher Scientific). DNA extracted from a blood sample of a VL patient and ultrapure water was included in all assays as positive and negative controls, respectively.
Determination of the avidity rate
The avidity rate (AR) was determined in serum samples from 44 of the 81 children from the AS group and 42 of the 43 children from the VL group that were positive to ELISA-rK39. The same conditions used for ELISA-rK39 were adopted with minor modifications: 50 μL of each serum sample diluted 1:100 in PBS-T-milk 1% were deposited in quadruplicate into the wells of the microplates, which were incubated for 1 h at 37°C. One duplicate of each sample was treated with 6 m urea in PBS for 5 min at room temperature. Then, the microplates were washed, and conjugate anti-human IgG linked to peroxidase (Sigma Chemical Co., US catalogue number: A8419) was added to each well. The plates were washed and a chromogenic solution (H2O2 + TMB – Sigma Chemical Co.) was added. The assay was incubated for 5 min at room temperature in the dark, and the enzymatic reaction was stopped. Samples that showed differences greater than 20% between the absorbance reading values of the duplicates were tested again. The AR was defined by the equation AR = U+/U− × 100, being: U+ = mean absorbance reading of the serum sample treated with 6 m urea solution and U− = mean absorbance reading of the untreated serum sample.
According to the protocol proposed by de Souza et al. (2005), samples that presented an AR lower than 40% were classified as samples of low avidity; samples with AR between 41 and 70% were classified as presenting mean avidity; those with AR greater than 70% were considered of high avidity.
Quantification of serum cytokine levels by flow cytometry
To measure the levels of the cytokines IL-2, IL-4, IL-6, IL-10, TNF, IFN-γ, IL-17A, IL-1β, IL-12p70 and the chemokine CXCL-8 (IL-8), the Human Th1/Th2/Th17 Cytokine CBA Kit (catalogue number: 560484) and the Human Inflammation CBA Kit (catalogue number: 551811) (BD Biosciences, San Jose, CA, USA) were used. The manufacturer's protocol was adapted as described by Peruhype-Magalhães et al. (2006). Samples of 81 asymptomatic, 43 VL and 98 uninfected children were tested. Twenty-five microlitre aliquots of sera and standards (supplied by the CBA kit) were mixed with 15 μL polystyrene microspheres coated with anti-IL-2, IL-4, IL-6, IL-10, TNF, IFN-γ, IL-17A, IL-1β, CXCL-8 and IL-12p70 antibodies and incubated for 90 min at room temperature. Next, 20 μL of developer reagent (a cocktail of human anti-cytokine monoclonal antibodies conjugated with phycoerythrin) were added and the tubes were incubated for 3 h at room temperature. The samples were washed with 500 μL of wash buffer solution, followed by centrifugation at 600 × g, for 10 min at room temperature. Then, the samples were immediately analysed on a Fortessa® LSR Flow Cytometer (BD). A total of 300 events/region (R1) were obtained for each biomarker evaluated. Data analysis was performed using the FCAP 3.0 Array software (BD). The 5-parameter curve fit (5PL) was used. This strategy allowed for extrapolation of values for sample intensities falling outside the limits of the standard curve. The improved accuracy of concentration estimates that can be obtained using the 5PL instead of the 4PL as a function of the asymmetry present in the data was studied. Thus, the theoretical limit of detection for the immunological biomarkers evaluated in this study was satisfactorily calculated. Circulating biomarkers concentrations were expressed as pg mL−1 or using a log scale, and the results were plotted in bar graphs presenting the medians with inter-quartile ranges.
Statistical analysis
The RI and AR were assessed using the Shapiro–Wilk normality test (W test) and correlated using the Pearson test. Similarly, the cytokine data were subjected to the normality test (W test) and compared using the Kruskal–Wallis test, followed by multiple comparison Dunn's test in the GraphPad Prism 7.0 software (GraphPad Prism, San Diego, CA, USA). The Pearson test was also performed to verify the correlation between AR and the symptom time when hospitalized of the VL patients. Statistically significant differences were considered when P < 0.05.
Logistic regression analysis of the categorized data (Table 1) identified a significant relationship for modelling between RI for the ELISA-rK39 (RI-rK39), AR, IL-17A, IFN-γ, TNF, IL-1β and CXCL-8 (P < 0.25). These pre-selected variables were included in the general logistic regression model using the Stepwise method. The biomarkers AR, IL-17A, IFN-γ and IL-1β were selected for the final model, and the odds ratios (OR) were calculated. The discriminant analysis was performed to classify these biomarkers, and the Classification and Regression Tree (CART) was used to organize the dataset into more homogeneous groups concerning the probability of the outcome being evaluated. The selection criteria for the best division were based on the higher homogeneity or the lower degree of impurity of the subsets.
Table 1.
Rules for categorizing each variable
| Variable/category | 0 | 1 | 2 | 3 |
|---|---|---|---|---|
| (>Minimum) | (>1st Quartile) | (>Median) | (>3rd Quartile) | |
| RI-SLA | 0 | 0.44 | 0.57 | 0.75 |
| RI-rK39 | 0 | 0.53 | 0.59 | 0.79 |
| AR | 0 | 44.79 | 58.87 | 69.04 |
| IL-17Aa | 0 | 0.1 | 2.69 | 5.71 |
| IFN-γa | 0 | 0.1 | 0.39 | 1.28 |
| IL-4a | 0 | 0.1 | 0.18 | 0.71 |
| IL-2a | 0 | 0.1 | 0.8 | 1.4 |
| IL-12p70a | 0 | 0.84 | 2.24 | 4.69 |
| TNFa | 0 | 0.01 | 0.1 | 0.33 |
| IL-10a | 0 | 1.52 | 2.85 | 6.07 |
| IL-6a | 0 | 1.75 | 3.41 | 6.22 |
| IL-1βa | 0 | 0.01 | 0.1 | 0.55 |
| CXCL-8a | 0 | 7.3 | 13.21 | 21.67 |
RI-SLA, Reactivity Index ELISA-SLA; RI-rK39, Reactivity Index ELISA-rK39.
Immunological biomarkers concentration are shown in pg ml−1.
Results
IgG antibody avidity in L. infantum-infected children
The 86 serum samples that presented a positive result for the ELISA-rK39 test were subjected to the avidity test of IgG antibodies. Of these samples, 44 belonged to children with asymptomatic infection by L. infantum and 42 belonged to children who were diagnosed with VL. The period between the onset of clinical manifestations and the moment of hospitalization ranged from 4 to 40 days in 39 patients (93%), and only three patients (7%) presented symptoms between 60 and 120 days. Twenty-nine samples from the AS group (66%) presented AR between 41 and 70% (Fig. 2A). High AR (above 70%) was observed in 27 children (64%) of the VL group (Fig. 2A).
Fig. 2.
IgG antibody avidity profile and correlation index in L. infantum-infected children. (A) Table presenting IgG antibody avidity profile. (B) Correlation analysis between the time of symptoms when hospitalized and the AR of IgG antibodies in the VL group. The results are shown in the scatter plot. Pearson's test was applied. (C) Comparative analysis between the time of clinical manifestations when hospitalized for patients who presented low (below 40%), average (41–70%) and high (above 70%) avidity. The results presented in the scatter plot highlight the median and number of days of clinical manifestations of the disease. The Kruskal–Wallis test was applied, followed by the Dunn multiple comparison test. (D) Correlation analysis between RI-rK39 and avidity in the AS and VL groups. The results are shown in the scatter plot. Pearson's test was applied.
The Pearson test was performed to verify the correlation between the time of symptoms when hospitalized and the AR of IgG antibodies in the VL group. The results showed a weak and non-significant correlation of 0.25 (P = 0.110) (Fig. 2B). AR of 21–100% was observed in patients who presented clinical manifestations of the disease between 4 and 40 days (Fig. 2B). The different groups of patients who presented low (below 40%), medium (between 41 and 70%) and high (above 70%) avidity presented a median of 10–20 days of clinical manifestations of the disease when hospitalized (Fig. 2C).
Indeed, Pearson's tests were performed to verify the correlations between RI-rK39 and AR in children from the AS and VL groups. In AS children, an r of −0.646 (P = 0.0001) was observed for the correlation RI-rK39/AR (Fig. 2D). In the VL group, an r of 0.823 (P = 0.0001) was observed for the same correlation (Fig. 2D). The data showed a negative correlation between RI-rK39/AR in children from the AS group and a strong positive correlation between RI-rK39/AR in the VL group (Fig. 2D).
Circulating profile of CXCL-8 chemokine and cytokines in L. infantum-infected children
The profile of circulating CXCL-8, IL-1β, IL-2, IL-6, IL-12p70, TNF, IFN-γ, IL-4, IL-10 and IL-17A was identified in uninfected and asymptomatic children living in an area endemic for VL and in children diagnosed with VL. Data analyses were performed in comparison with the NI group and between the AS and VL groups. The data showed a basal circulating level of CXCL-8 chemokine and pro-inflammatory cytokines IL-6, IL-1β, TNF and IFN-γ in AS children as observed in the NI group. Likewise, the modulatory IL-10 cytokine presented basal levels in the AS group (Fig. 3B). On the other hand, VL children presented high levels (P < 0.001) of circulating CXCL-8 chemokine and IL-6, IL-1β, TNF, IFN-γ and IL-10 cytokines compared to the AS and NI groups (Fig. 3A and B). An important highlight in our data was the increased circulating level of IL-17A (P < 0.05) in the AS group in comparison with VL patients (Fig. 3B). No statistically significant difference was observed for the pro-inflammatory IL-12p70, IL-2 and the modulatory IL-4 cytokines between the groups evaluated (data not shown).
Fig. 3.
Profile of pro-inflammatory and modulatory biomarkers in L. infantum-infected children. Circulating cytokines were analysed in serum samples of uninfected (NI = 98), asymptomatic (AS = 81) and children with classical visceral leishmaniasis (VL = 43) by flow cytometric CBA immunoassay. (A) Pro-inflammatory biomarkers CXCL8, IL-6, IL-1β, TNF and IFN-γ. (B) Modulatory biomarkers IL-10 and IL-17A. Circulating biomarkers concentrations were expressed as pg ml−1. Results were plotted in bar graphs highlighting the median and the interquartile ranges and presented on a log scale. The 95% CI of serum levels of circulating biomarkers in the NI group (basal production) are shown by the dashed rectangle. Statistically significant differences (P < 0.05) between the VL and the NI groups are shown by #. Statistically significant differences between the AS and VL groups are presented by connector lines and the significance level (P < 0.05 or P < 0.001) is indicated by * or ***, respectively.
Complementary analyses showed an increase of IL-17A levels in asymptomatic individuals that presented AR <40%. Conversely, decreased IL-17A levels were observed in children from the AS group presenting AR >70% (Fig. 4A).
Fig. 4.
AR, IL-17A, IFN-γ and IL-1β as hallmark predictors in the VL. (A) Cytokine profiles of different AR are shown in bar graphs highlighting the median and the interquartile ranges. Statistically significant differences (P < 0.05) between AR in the AS group are presented by connector lines. (B) Logistic regression analysis presenting an adjusted model that indicated the predictive biomarkers in VL. (C) Classification and Regression Tree (CART) of predictive biomarkers AR, IL-17A, IFN-γ and IL-1β between the AS (grey scale) and VL (black) groups. In the rectangles, the gradation of grey colour, from light to dark in the AS group, highlights the increase in the probability of developing VL; the ‘N’, with its respective percentage, indicates the number of children allocated to the group after the division of the antecedent node. To convert the standardized value of the biomarker to its corresponding serum, use Table 1 of standardization of the variables for multivariate analysis.
Identification of immunological biomarkers in L. infantum infection
The results of the logistic regression analysis indicated a substantial decrease of IL-17A in the VL group in comparison with the AS group (OR = 0.55, IC 95% 0.37–0.82) (P = 0.0039). Conversely, the results pointed to a relevant increase of the cytokines IFN-γ and IL-1β. According to the adjusted model, circulating levels of IFN-γ were almost three times higher in the VL group in comparison with the AS group (OR = 2.69, CI 95% 1.52–4.75) (P = 0.0001). Similarly, AR in the VL group was almost twice as high as in the AS group (OR = 1.84, CI 95% 1.27–2.67) (P = 0.0023) (Fig. 4B). In addition, the discriminant analysis presented high percentages of correct answers: 80 and 88% for the AS and VL groups, respectively, with an overall proportion of correctness of 83%; thus, indicating an important differentiation between the AS and VL groups using the biomarkers AR, IL-17A, IFN-γ and IL-1β.
The CART method created a classification rule for predictors AR, IL-17A, IFN-γ and IL-1β, which were previously categorized and selected in the logistic regression analysis between the AS and VL groups. The first initialization variable (‘root node’) was the AR. AR lower than 73.58% along with IFN-γ values lower than 0.48 pg mL−1, detected in 40 children, indicated a decision in favour of asymptomatic infection. For AR greater than73.58% accompanied by IFN-γ greater than 0.48 pg mL−1 and IL-1β greater than 0.075 pg mL−1, the decision favoured 15 children with VL. Among the AS and VL poles, it was possible to observe associations between the predictive biomarkers AR, IFN-γ, IL-17A and IL-1β. Overall, low AR and IFN-γ and high IL-17A values indicated the AS group, while elevated AR, IFN-γ and IL-1β values indicated the VL group (Fig. 4C).
Discussion
In face of the difficulties and challenges in the characterization and monitoring of L. infantum asymptomatic infection, this study aimed to evaluate the contribution of a combined analysis of the AR of anti-Leishmania IgG antibodies and circulating cytokine levels as potential biomarkers to support asymptomatic infection.
Our data showed a predominance of high avidity IgG antibodies (AR above 70% in 27 of 42 children) in patients with VL, albeit lacking correlation between AR and time of evolution of the disease. Redhu et al. (2006) used ELISA with LdrKE-16 antigen and reported AR above 70% in patients with a chronic disease who are unresponsive to treatment and AR lower than 70% in those with clinical manifestations under 6 months. The authors suggest that the test could be used to estimate the time and duration of infection by L. donovani. Nevertheless, it is important to point out that Redhu et al. (2006) evaluated VL caused by L. donovani, which leads to clinical manifestations and evolution of the disease different from that observed in VL caused by L. infantum. Furthermore, in contrast with the present study, Redhu et al. (2006) evaluated AR in adult patients. Perhaps, the use of a different species may be the reason for the absence of correlation observed in our study. A follow-up study performed in India reported that 38 out of 55 (69%) asymptomatic individuals (family members and neighbours) who were ELISA-rK39 positive developed VL in a period of 12 months (Singh et al., 2002).
On the other hand, our results are in better agreement with a study that evaluated anti-Leishmania IgG antibody AR in seropositive individuals with different clinical manifestations of the infection caused by L. infantum using Western blot. In that study, IgG antibodies with low and high avidity were detected in serum samples of patients collected before and after 6 months of specific treatment. High avidity IgG antibodies were predominant in individuals with VL, with only 10% of samples presenting low avidity IgG antibodies. These data are consistent with the long incubation period generally observed in the disease (Tiburcio et al., 2013).
Although our data showed that higher AR correlated with high RI obtained by ELISA-rK39 in children with VL (Fig. 2), these results must be analysed with caution. To the best of our knowledge, this is the first time that the measurement of AR is performed by ELISA-rK39. We used a protocol described for measuring AR in cutaneous leishmaniasis (de Souza et al., 2005). However, in contrast with cutaneous leishmaniasis and toxoplasmosis, VL is a disease of chronic course and it is difficult to define its acute period. Moreover, the immune humoral response intensity in VL infections is different from that observed in cases of toxoplasmosis. The AR assay is already well established for toxoplasmosis and when performed during the first 3 months of pregnancy can help to diagnose an acute infection (Gontijo da Silva et al., 2015). In the context of toxoplasmosis, the parasite causes a strong cellular response based on IFN-γ secreted by T lymphocytes (Filisetti and Candolf, 2004). On the other hand, in VL, a mixed inflammatory/regulatory cytokine profile is addressed by balanced levels of IFN-γ and IL-10 (Peruhype-Magalhães et al., 2006). Perhaps for this reason, VL is characterized by a polyclonal activation of B lymphocytes that generates high levels of circulating antibodies (Galvão-Castro et al., 1984). Therefore, it is possible that the high levels of circulating antibodies against rK39 may affect the AR results.
Very few studies have evaluated AR in asymptomatic Leishmania spp. infection. In the present study, only serum samples from asymptomatic VL children who were ELISA-rK39 positive were retested in the AR assay. Although ELISA-rK39 has enabled the detection of a higher number of asymptomatic VL individuals resident in endemic areas (Braz et al., 2002; Moreno et al., 2006, 2009; de Gouvêa Viana et al., 2008), it is important to highlight that the laboratory criteria to classify asymptomatic Leishmania spp. infection are not clearly defined.
In the AS group, we observed that low AR correlated with high RI, while high AR correlated with low RI (Fig. 2). Similarly, Tiburcio et al. (2013) showed a predominance of low avidity IgG antibodies in asymptomatic individuals and an increase in AR in a portion of these individuals, when assessed approximately 3 years after the initial evaluation. On the other hand, de Gouvêa Viana et al. (2008), Moreno et al. (2009) and dos Santos Marques et al. (2012) showed that asymptomatic individuals present low parasite load and low serum antibody levels, which tend to decrease over time. Therefore, one can suggest that the 11 children with asymptomatic infection who presented AR in the range of 14–45% and a RI-ELISA-rK39 >2 (ranging from 2.02 to 8.5) may have been in contact with the parasite recently but did not present the symptomatic disease.
Two children identified with asymptomatic infection presented AR of 45% and RI-ELISA-rK39 <2 (1.1 and 1.3). This could suggest that these individuals are going through a transition phase of the infection, in which the levels of antibodies in the serum are already low and the AR is in the threshold between the indexes considered low and high. Tiburcio et al. (2013) also reported a group of asymptomatic individuals who showed antibodies of low avidity even after the interval of 3 years and suggested that increased avidity over time occurs only in individuals that have developed the active disease. Thus, this group could present a greater risk of developing the disease under conditions of immunosuppression. Our results showed that children with asymptomatic infection present different AR that may be associated with the time of infection. No child identified with asymptomatic infection presented an AR above the 80% observed among the majority of VL children. Thus, we can conclude that the AR may be a potential serological marker to evaluate the likely evolution of infection in asymptomatic children. Nevertheless, despite all the evidence presented by these studies and by Tiburcio et al. (2013) that corroborate this hypothesis, additional studies are still required.
Our results showed that higher levels of CXCL-8, IL-6, IL-1β, TNF and IFN-γ associated with modulatory IL-10 cytokine in VL children. An elevation of serum levels of pro-inflammatory biomarkers associated with IL-10 cytokine has also been reported in other studies (Peruhype-Magalhães et al., 2005; Peruhype-Magalhães et al., 2006; Costa et al., 2012; Costa et al., 2013; Dos Santos et al., 2016). A cytokine storm was observed in VL children and corresponds to the immunological profile observed in the systemic inflammatory response syndrome, similar to what has already been described in the literature (Costa et al., 2013; Dos Santos et al., 2016). On the other hand, asymptomatic children presented a basal cytokine profile, similar to uninfected individuals. These data corroborate those obtained by different studies that evaluated the profile of circulating cytokines associated with the VL clinical form (Peruhype-Magalhães et al., 2006; Khoshdel et al., 2009; Costa et al., 2012). Given that these individuals lacked signs and symptoms of VL, it is possible to suggest that they were infected with Leishmania spp. at some point, but effectively controlled the infection and maintained the homoeostasis of the immune system, which could explain the presence of serum profile biomarkers similar to uninfected individuals (Peruhype-Magalhães et al., 2006). Therefore, we believe that the baseline profile of circulating biomarkers presented by the AS group characterizes the asymptomatic infection. Moreover, studies using antigen-stimulated cells have identified and suggested the use of biomarkers, such as CXCL-10 and CXCL9 chemokines (Ibarra-Meneses et al., 2017; Porcino et al., 2019) and IL-12, IL-2, IFN-γ, IL-17A, IL-22, IL-4 and IL-10 cytokines (Peruhype-Magalhães et al., 2005; Pitta et al., 2009; Romano et al., 2009; Ibarra-Meneses et al., 2017) for the detection of L. infantum-infected individuals. These studies clearly show that asymptomatic VL individuals have specific immune response profiles that differ from uninfected individuals living in areas endemic for VL and from patients with active VL. Interestingly, our data demonstrated that children with asymptomatic infection had higher serum levels of IL-17A in comparison with VL children. These results are in agreement with a study by Pitta et al. (2009), which showed that IL-17A, IL-22 and Th1 type cytokines play complementary roles in the development of a protective immune response against VL and that any changes in this mechanism could increase the risk of the disease. Furthermore, Pitta et al. (2009) reported that individuals who did not develop VL during an outbreak produced higher levels of IL-17A. This raises two questions: Could IL-17A be a modulator of the immune response and promotes a balance that is essential for the resolution of the infectious process in VL? Is IL-17A an important biomarker in VL?
In face of these results and questions, in the present study, we evaluated the clinical applicability of the biomarkers highlighted in the course of L infantum infection, and especially IL-17A, using mathematical modelling, a robust statistical process capable of identifying elusive differences not observed by conventional analyses. The decision tree proposed to classify the biomarkers AR, IL-17A, IFN-γ and IL-1β, previously categorized and selected in the logistic regression analysis, created a rule of association of these predictors between AS and VL individuals. The analysis using the decision tree showed that children presenting AR results lower than 2.5 (73.58%) and serum levels of IFN-γ lower than 2.5 (0.48 pg mL−1) are classified as asymptomatic. If the AR values are lower than 2.5 (73.58%) and IFN-γ serum levels are greater than 2.5 (0.48 pg mL−1), the serum levels of the cytokine IL-17A should be considered. If IL-17A levels are lower than 0.5 (0.05 pg mL−1), the child should be classified as belonging to the VL group, while IL-17A serum levels greater than 0.5 (0.05 pg mL−1) increase the chance of a child belonging to the AS group.
It was also observed that individuals with AR values higher than 2.5 (73.58%), IFN-γ serum levels greater than 2.5 (0.48 pg mL−1) and cytokine IL-1β levels greater than 1.5 (0.075 pg mL−1) are highly likely to belong to the VL group. In addition, in cases in which the dosage of cytokine IL-1β is lower than 1.5 (0.075 pg mL−1), the child will also be classified as belonging to the VL group. IL-1β is a pro-inflammatory cytokine, produced by innate immunity cells, especially phagocytes, which are effectively infected by parasites. A wide panel of immunological biomarkers gives strength to the proposal and a better understanding of the disease prediction.
In conclusion, the classification proposed in this study, using statistical approaches (logistic regression and CART), suggests a potential application of AR followed by cytokines analysis, especially IFN-γ, IL-17A and IL-1β, as a complementary tool for evaluation, characterization and monitoring of asymptomatic infection in children living in areas endemic for VL. Nevertheless, further studies are needed to validate the real applicability of this approach in the context of L. infantum infection, especially in adult and HIV-infected individuals and assess how cost-effective this approach would be in clinical practice.
Financial support
CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), Brasília, DF, Brazil (no. 448449/2014-5), Brazilian Ministry of Health/National Leishmaniasis Control Program/Fundo Nacional de Saúde (no. 25000•204799/2013-65), and Fundação de Amparo à Pesquisa de Minas Gerais – FAPEMIG, financial support Pesquisador Mineiro (PPM-00591-16). G.M.R. Cunha is grateful to Capes-Brazil (Coordenação de Aperfeiçoamento de Nível Superior) for a master fellowship. M. Carneiro is grateful to CNPq-Brazil (Conselho Nacional de Desenvolvimento Científico e Tecnológico) for the productivity research fellowships. E. Oliveira is supported by CNPq-Brazil (Conselho Nacional de Desenvolvimento Cientifico e Tecnológico, Proc. 301159/2016-5).
Ethical standards
The Research Ethics Committees of each institution (Universidade Federal de Minas Gerais, Belo Horizonte City Hall and Instituto René Rachou) approved the protocol for this study (CAAE: 12046113.0.0000.5149). Legal guardians of the children involved in this study were required to sign the Informed Consent Form before the clinical examination and sample collection. The children were granted medical care and treatment when necessary.
Conflict of interest
None.
References
- Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J and de Boer M (2012) Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 7, e35671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badaro R, Jones TC, Carvalho EM, Sampaio D, Reed SG, Barral A, Teixeira R and Johnson WD Jr (1986) New perspectives on a subclinical form of visceral leishmaniasis. Journal of Infectious Diseases 148, 1003–1011. [DOI] [PubMed] [Google Scholar]
- Badaró R, Benson D, Eulálio MC, Freire M, Cunha S, Netto EM, Pedral-Sampaio D, Madureira C, Burns JM, Houghton RL, David JR and Reed SG (1996) Rk39: a cloned antigen of Leishmania chagasi that predicts active visceral leishmaniasis. Journal of Infectious Diseases 173, 758–761. [DOI] [PubMed] [Google Scholar]
- Braz RF, Nascimento ET, Martins DR, Wilson ME, Pearson RD, Reed SG and Jerônimo SM (2002) The sensitivity and specificity of Leishmania chagasi recombinant K39 antigen in the diagnosis of American visceral leishmaniasis and in differentiating active from subclinical infection. American Journal of Tropical Medicine and Hygiene 67, 344–348. [DOI] [PubMed] [Google Scholar]
- Burns JM, Shreffler WG, Benson DR, Ghalibt HW, Badaro R and Reed S (1993) Molecular characterization of a kinesin-related antigen of Leishmania chagasi that detects specific antibody in African and American visceral leishmaniasis. Proceedings of the National Academy of Sciences of the USA 90, 775–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldas AJM, Silva DRC, Pereira CCR, Nunes PMS, Silva BP, Silva AAM, Barral A and Costa JML (2001) Infecção por Leishmania (Leishmania) chagasi Em crianças de uma área endêmica de leishmaniose visceral americana na Ilha de São Luís – MA, Brasil. Revista da Sociedade Brasileira de Medicina Tropical 34, 15–21. [DOI] [PubMed] [Google Scholar]
- Costa CH, Pereira HF, Pereira FC, Tavares JP, Araújo MV and Gonçalves MJ (1999) Is the household dog a risk factor for American visceral leishmaniasis in Brasil? Transactions of the Royal Society of Tropical 93, 464. [DOI] [PubMed] [Google Scholar]
- Costa CH, Stewart JM, Gomes RB, Garcez LM, Ramos PK, Bozza M, Satoskar A, Dissanayake S, Santos RS, Silva MR, Shaw JJ, David JR and Maguire JH (2002) Asymptomatic human carriers of Leishmania chagasi. American Journal of Tropical Medicine and Hygiene 66, 334–337. [DOI] [PubMed] [Google Scholar]
- Costa AS, Costa GC, Aquino DM, Mendonça VR, Barral A, Barral-Netto M and Caldas Ade J (2012) Cytokines and visceral leishmaniasis: a comparison of plasma cytokine profiles between the clinical forms of visceral leishmaniasis. Memórias do Instituto Oswaldo Cruz 107, 735–739. [DOI] [PubMed] [Google Scholar]
- Costa DL, Rocha RL, Carvalho RM, Lima-Neto AS, Harhay MO, Costa CH, Barral-Neto M and Barral AP (2013) Serum cytokines associated with severity and complications of Kala-azar. Pathogens and Global Health 107, 78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Rocha ICM, Dos Santos LHM, Coura-Vital W, da Cunha GMR, Magalhães FDC, da Silva TAM, Morais MHF, Oliveira E, Reis IA and Carneiro M (2018) Effectiveness of the Brazilian Visceral Leishmaniasis Surveillance and Control Programme in reducing the prevalence and incidence of Leishmania infantum infection. Parasites & Vectors 11, 586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gouvêa Viana L, de Assis TS, Orsini M, da Silva AR, de Souza GF, Caligiorne R, da Silva AC, Peruhype-Magalhães V, Marciano AP, Martins-Filho OA and Rabello A (2008) Combined diagnostic methods identify a remarkable proportion of asymptomatic Leishmania (Leishmania) chagasi carriers who present modulated cytokine profiles. Transactions of the Royal Society of Tropical Medicine and Hygiene 102, 548–555. [DOI] [PubMed] [Google Scholar]
- de Souza MA, da Sil AG, Afonso-Cardoso SR, Favoreto SJ and Ferreira MS (2005) Immunoglobulin isotype and IgG subclass profiles in American tegumentary leishmaniasis. Revista da Sociedade Brasileira de Medicina Tropical 38, 137–141. [DOI] [PubMed] [Google Scholar]
- Dos Santos PL, de Oliveira FA, Santos ML, Cunha LC, Lino MT, de Oliveira MF, Bomfim MO, Silva AM, de Moura TR, de Jesus AR, Duthie MS, Reed SG and de Almeida RP (2016) The severity of visceral leishmaniasis correlates with elevated levels of serum IL-6, IL-27 and sCD14. PLoS Neglected Tropical Diseases 10, e0004375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Santos Marques LH, Gomes LI, da Rocha IC, da Silva TA, Oliveira E, Morais MH, Rabello A and Carneiro M (2012) Low parasite load estimated by qPCR in a cohort of children living in urban area endemic for visceral leishmaniasis in Brazil. PLoS Neglected Tropical Diseases 6, e1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans TG, Teixeira MJ, McAuliffe IT, Vasconcelos IAB, Vasconcelos AW, Sousa QA, Lima JWO and Pearson RD (1992) Epidemiology of visceral leishmaniasis in Northeast Brazil. Journal of Infectious Diseases 166, 1124–1132. [DOI] [PubMed] [Google Scholar]
- Filisetti D and Candolf E (2004) Immune response to Toxoplasma gondi. Annali dell'Istituto Superiore di Sanità 40, 71–80. [PubMed] [Google Scholar]
- Galvão-Castro B, Sá Ferreira JÁ, Marzochi KF, Marzoch MC, Coutinho SG and Lambert PH (1984) Polyclonal B cell activation, circulating immune complexes and autoimmunity in human American visceral leishmaniasis. Clinical Experimental Immunology 56, 58–66. [PMC free article] [PubMed] [Google Scholar]
- Gontijo da Silva M, Clare Vinaud M and de Castro AM (2015) Prevalence of toxoplasmosis in pregnant women and vertical transmission of Toxoplasma gondii in patients from basic units of health from Gurupi, Tocantins, Brazil, from 2012 to 2014. PLoS ONE 10, e0141700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho M, Leeuwenburg J, Mbugua G, Wamachi A and Voller A (1983) An enzyme-linked immunosorbent assay (ELISA) for field diagnosis of visceral leishmaniasis. American Journal of Tropical Medicine and Hygiene 32, 943–946. [DOI] [PubMed] [Google Scholar]
- Ibarra-Meneses AV, Sanchez C, Alvar J, Moreno J and Carrillo E (2017) Monocyte chemotactic protein 1 in plasma from soluble leishmania antigen-stimulated whole blood as a potential biomarker of the cellular immune response to Leishmania infantum. Frontiers in Immunology 29, 1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerônimo SM, Teixeira MJ, Sousa AD, Thielking P, Pearson RD and Evans TG (2000) Natural history of Leishmania (Leishmania) chagasi infection in northeastern Brazil: long-term follow-up. Clinical Infectious Diseases 30, 608–609. [DOI] [PubMed] [Google Scholar]
- Khoshdel A, Alborzi A, Rosouli M, Taheri E, Kiany S and Javadian MH (2009) Increased levels of IL-10, IL-12, and IFN-gamma in patients with visceral leishmaniasis. Brazilian Journal of Infectious Diseases 13, 44–46. [DOI] [PubMed] [Google Scholar]
- Moreno EC, Melo MN, Lambertucci JR, Serufo JC, Andrade AS, Antunes CM, Genaro O and Carneiro M (2006) Diagnosing human asymptomatic visceral leishmaniasis in an urban area of the State of Minas Gerais, using serological and molecular biology techniques. Revista da Sociedade Brasileira de Medicina Tropical 39, 421–427. [DOI] [PubMed] [Google Scholar]
- Moreno EC, Gonçalves AV, Chaves AV, Melo MN, Lambertucci JR, Andrade AS, Negrão-Corrêa D, Figueiredo Antunes CM and Carneiro M (2009) Inaccuracy of enzyme-linked immunosorbent assay using soluble and recombinant antigens to detect asymptomatic infection by Leishmania infantum. PLoS Neglected Tropical Diseases 3, 536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso O, Sommer P, Drouet E, Cotte L, Neyra M, Grimaud J-A and Chevallier M (1996) In situ detection of human cytomegalovirus DNA in gastrointestinal biopsies from AIDS patients by means of various PCR-derived methods. Journal of Virological Methods 56(2), 125–137. 10.1016/0166-0934(95)01892-1 [DOI] [PubMed] [Google Scholar]
- Peruhype-Magalhães V, Martins-Filho OA, Prata A, Silva L, Rabello A, Teixeira-Carvalho A, Figueiredo RM, Guimarães-Carvalho SF, Ferrari TC and Correa-Oliveira R (2005) Immune response in human visceral leishmaniasis: analysis of the correlation between innate immunity cytokine profile and disease outcome. Scandinavian Journal of Immunology 62, 487–495. [DOI] [PubMed] [Google Scholar]
- Peruhype-Magalhães V, Martins-Filho OA, Prata A, Silva LdA, Rabello A, Teixeira-Carvalho A, Figueiredo RM, Guimarães-Carvalho SF, Ferrari TC, Van Weyenbergh J and Correa-Oliveira R (2006) Mixed inflammatory∕regulatory cytokine profile marked by simultaneous raise of interferon-gamma and interleukin-10 and low frequency of tumour necrosis factor-alpha(+) monocytes are hallmarks of active human visceral leishmaniasis due to Leishmania chagasi infection. Clinical & Experimental Immunology 146, 124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitta MG, Romano A, Cabantous S, Henri S, Hammad A, Kouriba B, Argiro L, el Kheir M, Bucheton B, Mary C, El-Safi SH and Dessein A (2009) IL-17 and IL-22 are associated with protection against human kala azar caused by Leishmania donovani. The Journal of Clinical Investigation 119, 2379–2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcino GN, Carvalho KSS, Braz DC, Costa Silva V, Costa CHN and de Miranda Santos IKF (2019) Evaluation of methods for detection of asymptomatic individuals infected with Leishmania infantum in the state of Piauí, Brazil. PLoS Neglected Tropical Diseases 13, e0007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redhu NS, Dey A, Balooni V and Singh S (2006) Use of immunoglobulin G avidity to determine the course of disease in visceral and post-kala-azar dermal leishmaniasis patients. Clinical and Vaccine Immunology 13, 969–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues-Neto JF, Monteiro GR, Keesen TSL, Lacerda HG, Carvalho EM and Jeronimo SMB (2018) CD45RO + T cells and T cell activation in the long-lasting immunity after Leishmania infantum infection. The American Journal of Tropical Medicine and Hygiene 98, 875–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano A, Cabantous S, Henri S, Hammad A, Kouriba B, Argiro L, el Kheir M, Bucheton B, Mary C, El-Safi SH and Dessein A (2009) IL-17 and IL-22 are associated with protection against human kala-azar caused by Leishmania donovani. Journal of Clinical Investigation 119, 2379–2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero GA and Boelaert M (2010) Control of visceral leishmaniasis in Latin America a systematic review. PLoS Neglected Tropical Diseases 4, e584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Kumari V and Sing N (2002) Predicting kala-azar disease manifestations in asymptomatic patients with latent Leishmania donovani infection by detection of antibody against recombinant K39 antigen. Clinical and Diagnostic Laboratory Immunology 9, 568–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiburcio MG, Anversa L, Kanunfre KA, Ferreira AW, Rodrigues Júnior V and Silva LdA (2013) Anti-Leishmania infantum IgG antibody avidity in visceral leishmaniasis. Clinical and Vaccine Immunology 20, 1697–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eys GJJM, Schoone GJ, Kroon CM and Ebeling SB (1992) Sequence analysis of small subunit RNA genes and its use for detection and identification of Leishmania parasites. Molecular and Biochemical Parasitology 51, 133–142. [DOI] [PubMed] [Google Scholar]
- WHO (2019) Leishmaniasis Geneva: World Health Organization. WHO. https://www.who.int/leishmaniasis/burden/en/. Last access September 10 2019. [Google Scholar]
- Wortmann G, Sweeney C, Houng HS, Aronson N, Stiteler J, Jackson J and Ockenhouse C (2001) Rapid diagnosis of leishmaniasis by fluorogenic polymerase chain reaction. American Journal of Tropical Medicine and Hygiene 65, 583–587. [DOI] [PubMed] [Google Scholar]