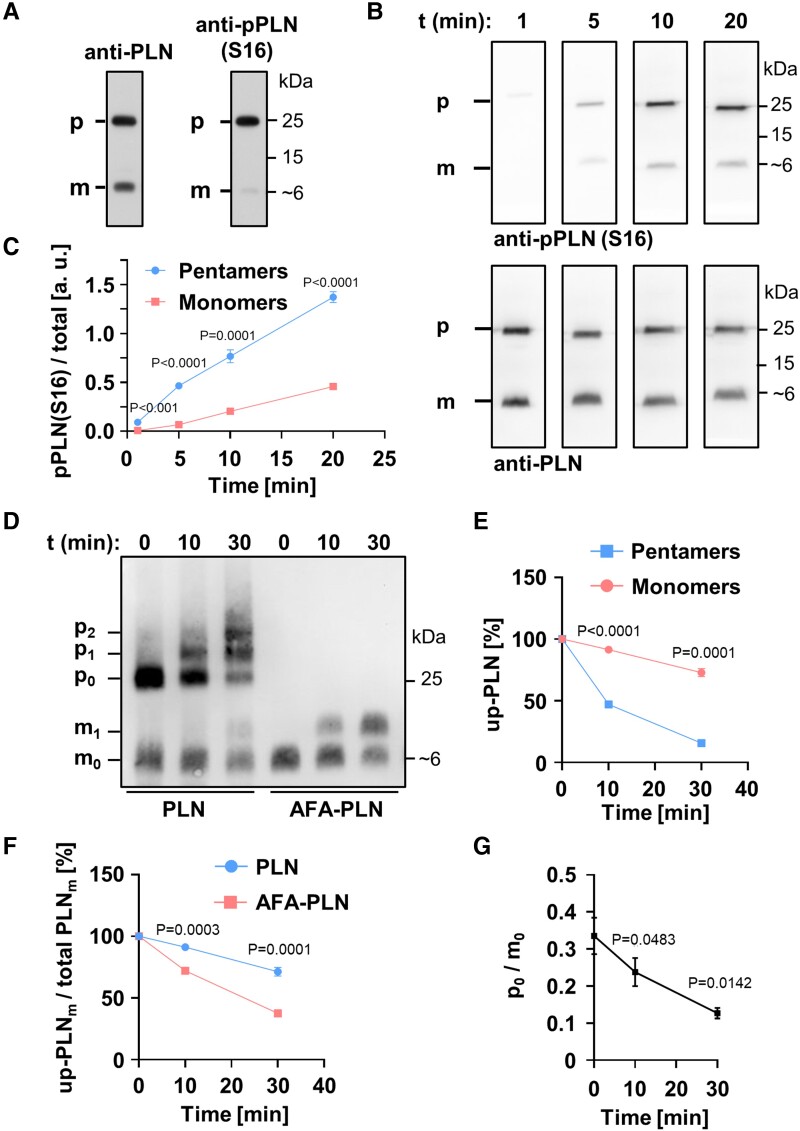
Figure 1.
Phospholamban (PLN) pentamers are excellent protein kinase A (PKA) substrates. (A) SDS-PAGE and western blot using mouse heart lysates probed with antibodies against total PLN (left) or PLN phosphorylated at S16 (right). (B) Far western kinase assay; phosphorylation of separated and fixed pentamers and monomers with PKA; antibodies as in (A). Heart lysates were dephosphorylated prior to separation and fixation of PLN monomers and pentamers on polyvinylidene difluoride membrane. After renaturation, pentamers and monomers were phosphorylated with PKA for 1, 5, 10, or 20 min. (C) Quantification of (B); shown are the mean signal intensities of PKA-dependent monomer/pentamer phosphorylation at S16. Phosphorylation signals of monomers and pentamers were normalized to the respective total PLN signal intensities. Mean ± SEM, N = 8 hearts, two-way ANOVA followed by Sidak’s multiple comparisons test. (D, E) In vitro phosphorylation of synthetic PLN (adjusted for equal monomer amounts) by PKA for 0, 10, and 30 min in solution followed by western blot using Phos-tag™-gels and anti-PLN antibodies; mx, px: Monomers (pentamers) with x = 0, 1, 2 phosphate group(s); up-PLN, unphosphorylated PLN. Note the strong phosphorylation of pentamers (p1 + p2) compared to monomers (m1) for wild-type PLN (PLN). Mean ± SEM, N = 6, pentamers and monomers compared by two-way ANOVA followed by Sidak’s multiple comparisons test. (F) PLN monomer depletion during PKA-dependent phosphorylation comparing wild-type PLN and AFA-PLN as shown in (D); Mean ± SEM, N = 6, two-way ANOVA followed by Sidak’s multiple comparisons test. (G) Molar substrate ratios of unphosphorylated pentamers (p0) and monomers (m0) as quantified from (D). Mean ± SEM, N = 6, repeated measures one-way ANOVA followed by Tukey’s multiple comparisons test vs. t0.