Figure 1.
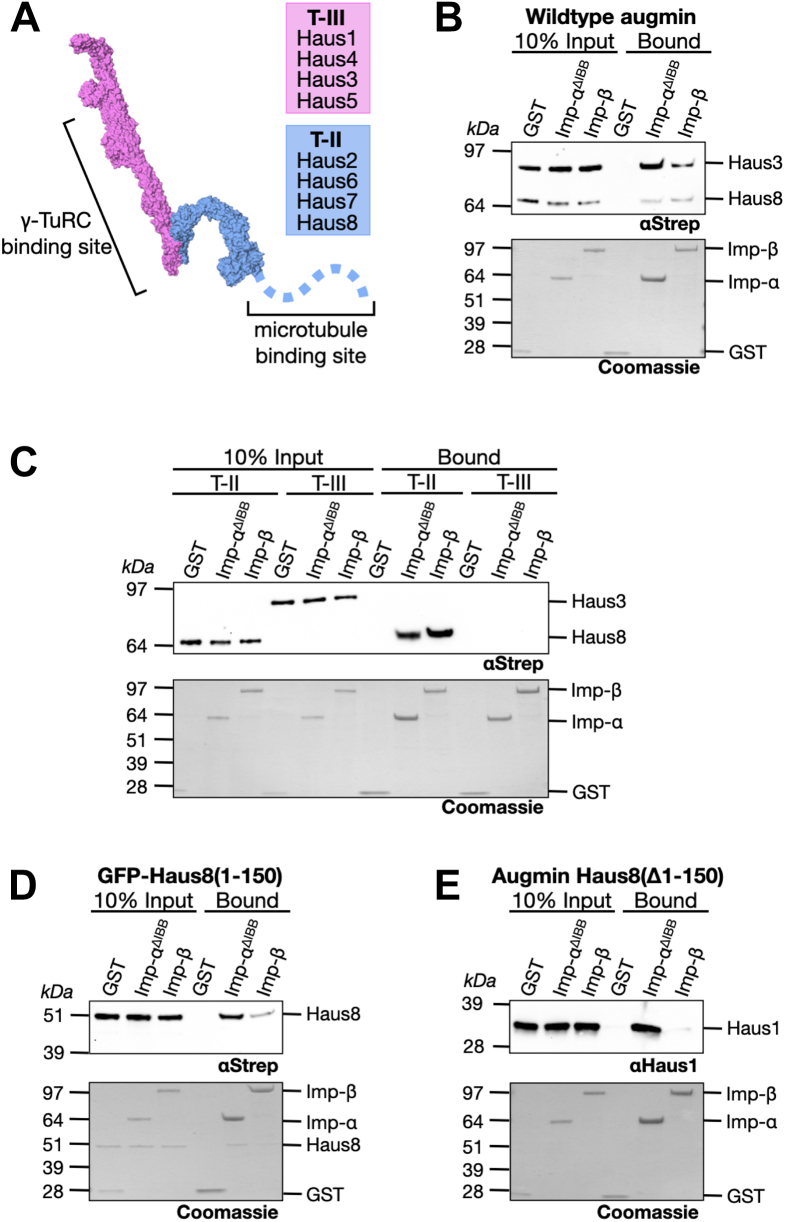
Augmin binds to importins.A, structure of the augmin complex, broken into the γ-TuRC binding T-III (pink), comprised of subunits Haus1 and Haus3–5, and the MT-binding T-II (blue), comprised of subunits Haus2 and Haus6–8. The disordered N terminus of Haus8 (shown as a dashed blue line) contains the primary MT-binding site. Structure of Xenopus augmin was taken from Ref. (39). B, glutathione beads bound to either GST (control), GST-importin-αΔIBB, or GST-importin-β were incubated with full-length augmin, then both the input and bound fraction were Western blotted for intact augmin complexes using an antibody against the Strep-tagged subunits Haus3 (T-III) and Haus8 (T-II). Below, GST and GST-importin loading was demonstrated by Coomassie stain. C, as in (B), importin-bound beads were incubated with augmin, either T-III or T-II, and binding of intact augmin subcomplex was detected via Western blot against the Strep-tagged subunits Haus3 and Haus8. D, Haus81–150 (fused to an N-terminal Strep-tagged GFP) was incubated with importin-bound beads, and binding was detected via Western blot. E, augmin complex lacking the N-terminal 150 residues of Haus8 was incubated with importin-bound beads, and binding of intact augmin complex was detected via Western blot against augmin subunit Haus1. γ-TuRC, γ-tubulin ring complex; GST, glutathione-S-transferase; IBB, importin-β binding; MT, microtubule; T-III, tetramer III.