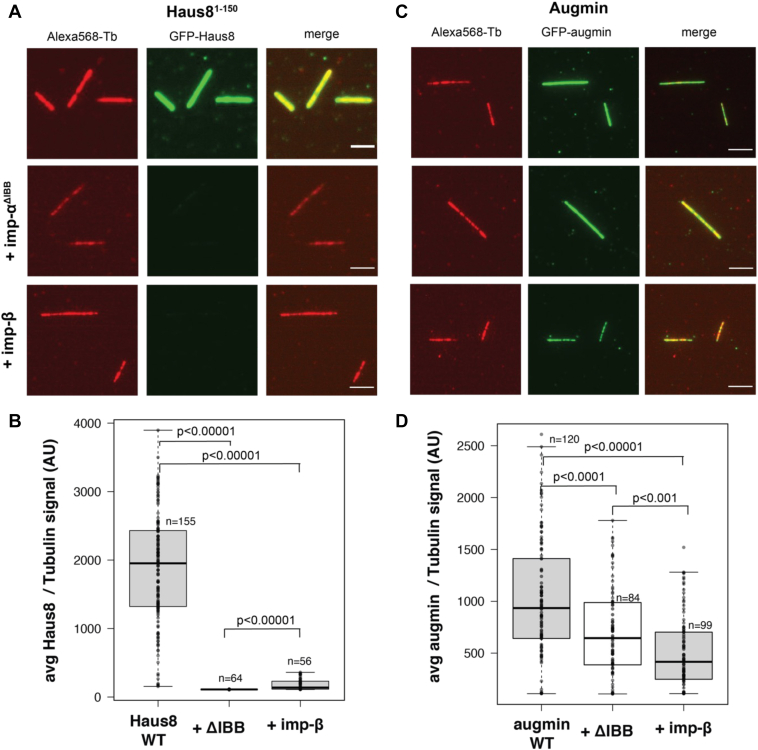
Figure 3.
Importins regulate augmin binding to MTs.A, WT GST-GFP-Haus8 localizes strongly to GMPCPP-stabilized MT seeds in vitro (top row), as visualized by TIRF microscopy. In the presence of importin-αΔIBB (middle row) or importin-β (bottom row), binding of Haus8 to MTs is diminished. This is quantified in B. B, boxplot of average GFP-Haus8 signal relative to the average tubulin signal, where each marker represents a single MT from the experiment shown in F. The total number of MTs (n) was collected from two replicates. The boxes extend from the 25th to 75th percentile, and the upper and lower bars represent the minimum and maximum, respectively. p Values were calculated from independent t tests. C, WT GFP-labeled augmin localized to GMPCPP-stabilized MT seeds in vitro (top row), as visualized by TIRF microscopy. In the presence of importin-αΔIBB (middle row) or importin-β (bottom row), binding of augmin to MTs is decreased but not eliminated. This is quantified in D. D, boxplot of average GFP-augmin signal relative to the average tubulin signal, where each marker represents a single MT from the experiment shown in F. The total number of MTs (n) was collected from two replicates using two independent augmin preparations. Center lines show the medians; box limits indicate the 25th and 75th percentiles as determined by R software; whiskers extend 1.5 times the interquartile range from the 25th and 75th percentiles. p Values were calculated from independent t tests. In A and C, images belonging to the same experiment were contrast matched. To compare Haus8 fluorescence intensity across experiments, the intensity was normalized with respect to the tubulin signal. Scale bars correspond to 5 μm. GST, glutathione-S-transferase; IBB, importin-β binding; MT, microtubule; TIRF, total internal reflection fluorescence.