FIGURE 1.
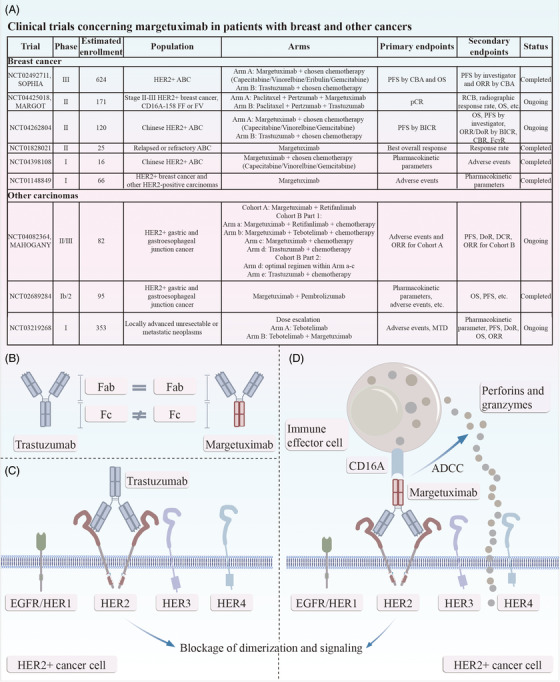
(A) Clinical trials concerning margetuximab in patients with breast and other cancer. (B) Margetuximab shares epitope specificity with trastuzumab, but with a modification of the Fc domain: five amino acids are altered from wild‐type immunoglobulin G1 to improve binding affinity for CD16A (FcγRIIIA), a stimulatory FcγR, and to reduce binding affinity for CD32B (FcγRIIB), an inhibitory FcγR. (C) Trastuzumab inhibits dimerization of HER2, thus downregulating downstream signaling, which induces the proliferation, cell‐cycle progression, survival, and invasiveness of cancer cells. (D) The Fc engineering of margetuximab leads to an enhanced ADCC activation against HER2‐positive cancer cells. ABC, advanced breast cancer; ADCC, antibody‐dependent cell‐mediated cytotoxicity; BICR, blinded independent central review; CBA, central blinded analysis; CBR, clinical beneficial rate; DCR, disease control rate; DoR, duration of response; EGFR, epidermal growth factor receptor; FcγR, Fcγ receptor; HER, human epidermal growth factor receptor; MTD, maximum tolerated dose; OS, overall survival; ORR, objective response rate; pCR, pathological complete response; PFS, progression‐free survival; RCB, residual tumor burden.
