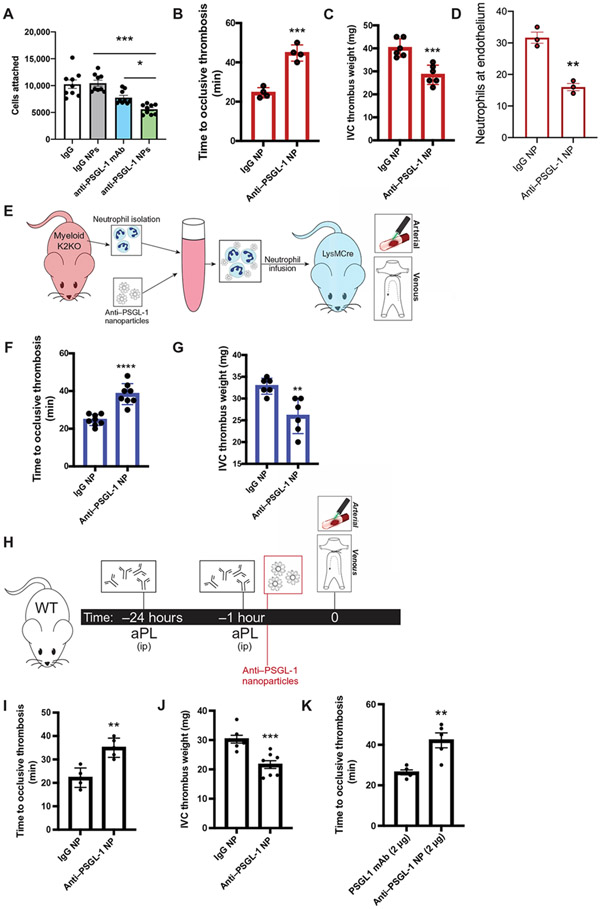
Fig. 7. Targeting PSGL-1 clustering in activated neutrophils with decorated nanoparticles protects against arterial and venous thrombosis.
(A) Cell attachment quantification from in vitro microfluidic assay is shown. K2KO neutrophils were preincubated with control IgG, anti–PSGL-1 antibody, control IgG NP, or anti–PSGL-1 NP before flowing through chambers (n = 9 trials). (B and C) The extent of thrombosis in K2KO mice treated with IgG or anti–PSGL-1–coated NP was compared for both (B) arterial and (C) venous models (n = 4 arterial and n = 6 venous). (D) Quantification of neutrophils along vessel walls early after carotid artery injury is shown for K2KO mice treated with either IgG (n = 3) or anti–PSGL-1 (n = 3) NPs. (E) The experimental design for adoptive neutrophil transfer is shown. Neutrophils from K2KO mice were incubated with IgG or anti–PSGL-1 NPs and transferred into LysM mice before thrombosis. (F and G) K2KO neutrophils preincubated with either IgG or anti–PSGL-1 NPs were infused into LysM mice before (F) carotid artery injury or (G) IVC ligation (n = 6 to 8). (H) An experimental timeline is shown depicting treatment of aPL-injected WT mice with either IgG or anti–PSGL-1 NPs before thrombosis induction. (I and J) The effect of anti–PSGL-1 NP treatment against aPL-induced (I) arterial and (J) venous thrombosis was measured (n = 5 to 8). (K) Lower doses of anti–PSGL-1 antibody were compared in an aPL-injected carotid artery injury model (n = 5). Anti–PSGL-1 was administered either alone or in NP formulation at concentrations of 2 μg. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 by unpaired, two-tailed Student’s t test.