Fig. 5.
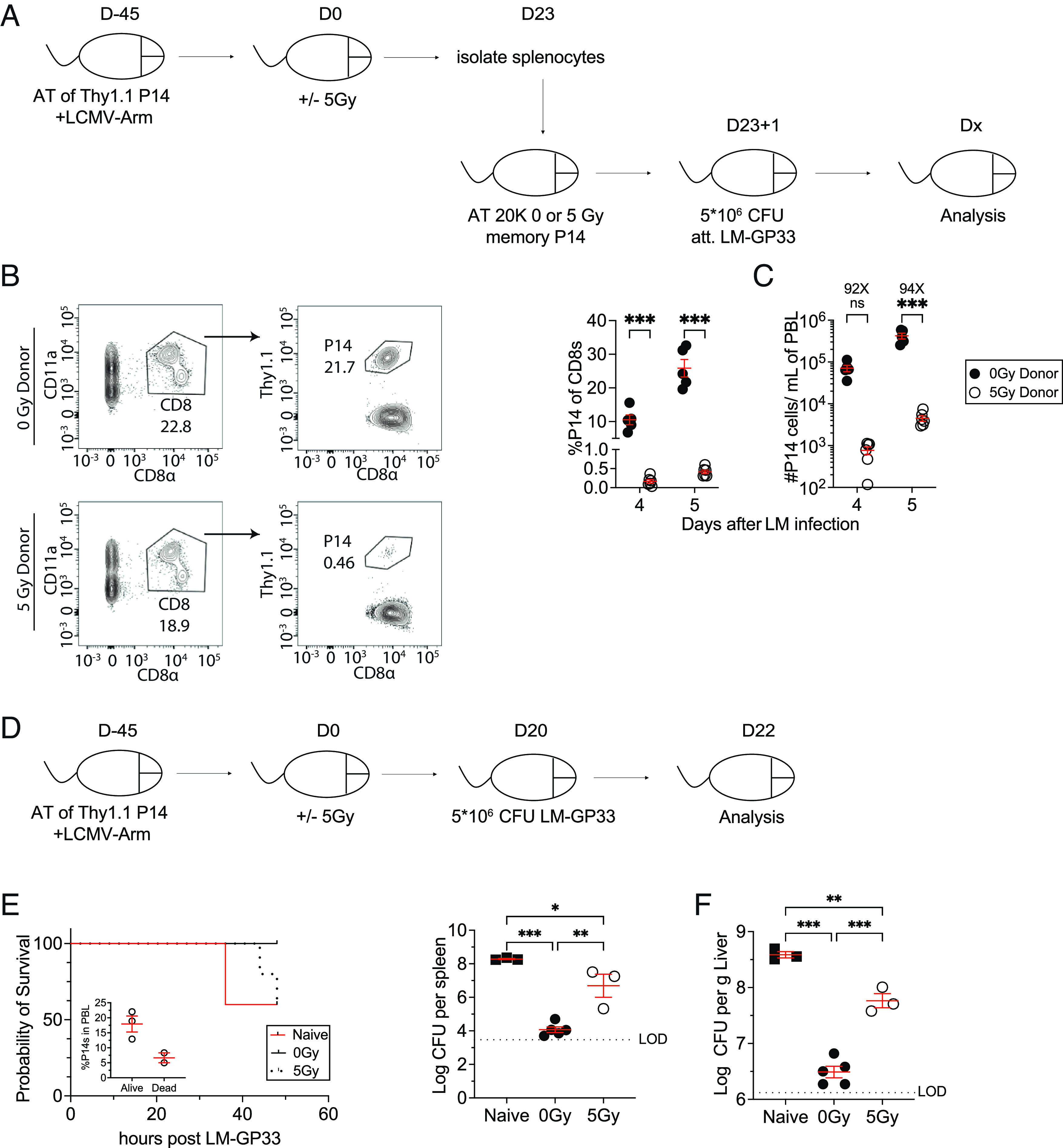
WBI substantially impairs the ability of memory CD8 T cells and immunized hosts to respond to rechallenges. (A) Experimental design: 104 naive Thy1.1+ P14 CD8+ T cells were adoptively transferred into Thy1.2+ naïve hosts, followed by LCMV-Arm infection to generate memory P14 CD8 T cells. Forty-five days later, the memory P14 chimeric mice were either exposed to mock or 5Gy WBI. Twenty-three days after mock or 5Gy WBI, splenocytes from 0Gy and 5Gy mice were isolated and 2 × 104 memory P14 CD8 T cells were then transferred into naïve mice. The P14-recipient mice were then infected with attenuated L. monocytogenes expressing GP33-41 (LM-GP33). (B) Representative gating of 0Gy donor (Top) and 5Gy donor (Bottom) P14 CD8 T cells in recipient mice 5 d after attenuated LM-GP33 challenge. (C) Frequency of donor P14 CD8 T cells of total recipient CD8 T cells (Left) and the number of donor P14 CD8 T cells (Right) 4 and 5 d after attenuated LM-GP33 challenge assessed in blood. (D) 104 naive Thy1.1+ P14 CD8+ T cells were adoptively transferred into Thy1.2+ naïve hosts, followed by LCMV-Arm infection to generate memory P14 CD8 T cells. Forty-five days later, the memory P14 chimeric mice were either exposed to mock or 5Gy WBI. These mice as well as a group of naïve mice with no previous pathogen exposure were then infected with 5 × 106 CFU of virulent L. monocytogenes expressing GP33-41 (LM-GP33) 20 d after mock or 5Gy WBI. (E) Mortality of mice after infection. (F) CFUs of LM-GP33 per gram of the liver (Left) and spleen (Right) 2 d after infection. All data are representative of at least two independent experiments with four to five mice per group. *=P < 0.05, **=P < 0.01, ***=P < 0.001. Error bars represent SEM.
