Abstract
DNA mismatch repair protein deficiency (MMRd) in endometrial carcinoma is associated with the risk of Lynch syndrome and response to immune checkpoint inhibitors. It is also related to microsatellite instability and corresponds to a molecular subtype of endometrial tumor with an unclear prognosis. Here, we evaluated the clinicopathological characteristics and prognosis of 312 consecutive endometrial carcinoma cases submitted to complete surgical staging at a single institution. We compared MMRd and mismatch repair protein–proficient (MMRp) tumors and examined the effects of the MMR protein loss type (MLH1/PMS2 vs. MSH2/MSH6) and influence of L1CAM and p53 expression. The median follow-up period was 54.5 (range, 0–120.5) months. No difference was observed between MMRd [n = 166 (37.2%)] and MMRp [n = 196 (62.8%)] cases in terms of age, body mass index, FIGO stage, tumor grade, tumor size, depth of myometrial infiltration, or lymph node metastasis. More MMRd than MMRp tumors had endometrioid histology (87.9% vs. 75.5%) and despite MMRd had more lymphovascular space invasion (LVSI; 27.2% vs. 16.9%), they presented fewer recurrences and no difference in lymph node metastasis and disease-related death. Relative to those with MLH1/MSH6 loss, tumors with MSH2/MSH6 loss were diagnosed at earlier FIGO stages, were smaller, and had less ≥50% myometrial invasion, LVSI and lymph node metastasis. Outcomes, however, did not differ between these groups. L1CAM positivity and mutation-type p53 expression were more common in MMRp than in MMRd tumors and did not differ between the MLH1/PMS2 and MSH2/MSH6 loss groups. In the entire cohort, L1CAM and mutation p53 expression were associated with worse prognosis, but only non-endometrioid histology, FIGO stage III/IV, and deep myometrial infiltration were significant predictors. In the subgroup of endometrioid carcinomas, only FIGO stage III/IV was associated with poor outcomes. The risk of lymph node metastasis was associated with tumor size, non-endometrioid histology, and multifocal LVSI. For MMRd tumors, only tumor size and myometrial invasion depth were predictive of lymph node involvement. In our cohort, MMRd tumors were associated with greater recurrence-free, but not overall, survival. The precise identification of MMRd status, present in a substantial proportion of endometrial cancer cases, is a challenge to be overcome for proper patient management. MMRd status serves as a marker for Lynch syndrome, and a significant number of these tumors are high risk and candidate to immunotherapy.
Keywords: Endometrial carcinoma, DNA mismatch repair protein, L1CAM, p53, Prognosis
1. Introduction
The incidence of endometrial cancer has grown in recent decades, in developed countries such as the USA and developing countries such as Brazil [1,2]. Contributing factors include obesity and increased life expectancy. In general, cancer survival rates have tended to increase, except for cancers involving the corpus uteri and cervix, due to the relative ineffectiveness of available treatment options [1].
Endometrial cancer was long classified as endometrioid and non-endometrioid, but in 2013 The Cancer Genome Atlas (TCGA) research network identified four main subtypes of genomic somatic alteration among endometrioid and serous endometrial carcinomas [3]. This group reported that tumors with pathogenic polymerase epsilon (POLE) mutations and those exhibiting high microsatellite instability (MSI-H) had the greatest tumor mutation burden and immune activation [3]. Those harboring POLE mutations are referred to as ultramutated because they have the largest number of mutations. They have an excellent prognosis, independent of any other feature. Tumors exhibiting MSI, which comprise approximately 30% of the cases, are referred hypermutated [3]. Both POLEmut and MSI-high (MSI-H) are tumors with the highest tumor mutation burden and immune activation. The remaining tumors, with fewer mutations, are classified based on the number of somatic copy number (CN) alterations. CN-high tumors, including all serous and some high grade endometrioid, have the worst prognosis; they exhibit chromosomal instability and recurrent TP53 mutations [3]. CN-low tumors, most of which are endometrioid, have elevated RAD50 expression, associated with DNA repair, which may contribute to chromosomal stability [3]. The translation of TCGA classification to practice became feasible with the development of the Proactive Molecular Risk Classifier for Endometrial Cancer tool [4]. The use of this classifier involves the sequencing POLE exons 9–14 and immunohistochemistry of the mismatch repair (MMR) proteins MLH1, MSH2, MSH, and PMS2 for the identification of POLEmut and MSI-H tumors, respectively. After exclusion of these two groups, p53 immunohistochemical analysis is performed to classify the CN status as high (mutation-type p53, p53mut) or low (nonspecific molecular profile, wild-type p53, p53w). Despite the robust prognostic implications of such molecular classification, particularly for POLE-mutated and p53mut groups, clinical risk stratification is still based on classical clinicopathological characteristics, as outlined in the recommendations of the 2016 European Society for Medical Oncology, European Society of Gynaecological Oncology and European Society for Radiotherapy and Oncology (ESMO-ESGO-ESTRO) recommendations [5]. The current ESMO recommendations include molecular classification and the consideration of POLE mutation and p53mut to prevent over- and under-treatment, respectively [6]. Classical pathological characteristics, such as the histological type, tumor grade, lymphovascular space invasion (LVSI), depth of myometrial infiltration, and lymph node involvement, remain discriminatory for tumors with nonspecific molecular profile and those with mismatch repair deficiency (MMRd), but these tumor types are very heterogeneous and their classification would be improved by the consideration of additional prognostic factors. One such factor is the expression of L1 Cell Adhesion Molecule (L1CAM), a transmembrane glycoprotein of the immunoglobulin superfamily, that is involved in the process of epithelial-mesenchymal transition (EMT) and is expressed by different aggressive tumors types [[7], [8], [9]].
MSI-H tumors have loss-of-function in the DNA of MMR genes (MLH1, MSH2, MSH6, and PMS2), either somatic or germline, with the latter causing Lynch syndrome (LS). About 17% of MSI-H cases are associated with LS, corresponding to about 4% of all endometrial cancer [10]. In most endometrial cancers with MMRd, the loss of protein function is secondary to MLH1 hypermethylation [11]. MMRd tumors are more sensitive to radiation and immunotherapy, with questionable benefit from chemotherapy [[12], [13], [14]]. The mechanisms by which MMR proteins defects determine MSI possibly differ based on whether the loss is due to MLH1 hypermethylation, somatic mutations, or germline mutations [15,16]. The MSI-H/MMRd status is associated with a high neoantigen load and immune activation, resulting in greater susceptibility to checkpoint inhibitors; it thus serves as a biomarker indicating selection for such treatment [17,18].
In this study we compared the clinicopathological characteristics and prognoses of MMRd and mismatch repair-proficient (MMRp) endometrial cancers. We sought to identify any difference between these groups, the MMR protein loss type (MLH1/PMS2 and MSH2/MSH6), and the influences of L1CAM and p53.
2. Methods
This study was approved by the Scientific Committee of the Department of Pathology of Faculdade de Medicina da Universidade de Sao Paulo and by the Ethics Committee for Research Projects of the Hospital das Clınicas da Faculdade de Medicina da Universidade de Sao Paulo (Comissao de Etica para Analise de Pesquisa - CAPPesq) (no. 351/16), and by Plataforma Brasil (CAAE 59579616.8.0000.0065). It complied with the ethical precepts proposed by the legislation in force in Brazil (R466/2012).
We retrospectively enrolled consecutive patients with endometrial carcinoma diagnoses confirmed between January 2011 and December 2017, who underwent complete surgical staging at the Instituto do Cancer do Estado de Sao Paulo (Sao Paulo, Brazil), a public teaching hospital, and were referred for cancer treatment. Data on patient age, family history of cancer, body mass index (BMI), International Federation of Gynecology and Obstetrics (FIGO) staging, primary treatment, tumor size (in centimeters), lymph node status, and follow-up were retrieved form the medical records. Tumors were classified according the 2020 World Health Organization (WHO) criteria and graded using the FIGO two-tiers system [19]. For statistical purposes histological types were grouped into endometrioid and non-endometrioid categories (Fig. 1A). For the same reasons, FIGO I and II were grouped as early stage, and III and IV as advanced. The same pathologist (FNA) reviewed all cases, determining the depth of myometrial infiltration (less than 50% or equal or more than 50%); presence of the microcystic, elongated, and fragmented pattern of myoinvasion [20]; and LVSI [no emboli, focal (just one peritumoral focus), or multifocal (four or more foci on one slide, or multiple foci in different slides)] [21]. For statistical purposes and as recommended by ESMO [6], LVSI was dichotomized as none/focal and multifocal) [21].
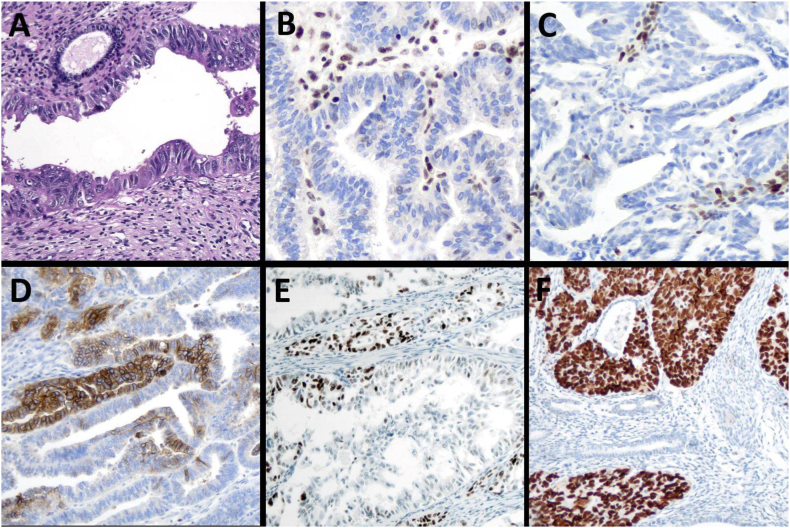
Fig. 1.
Representative histological and immunohistochemical images of endometrial carcinoma. A) non-endometrioid histological type showing a neoplastic gland of a serous endometrial carcinoma adjacent to a normal endometrial gland (original magnification ×200). B) MLH1: loss of immunoexpression in tumor cells and normal expression in stroma (original magnification ×200). C) MSH6: loss of expression in tumor cells (original magnification ×200). D) L1CAM-positive: membranous staining in more than 10% of tumor cells (original magnification ×100). E) wild-type p53 expression with some tumor cells showing nuclear staining (original magnification ×100). F) mutant-type p53 showing strong and diffuse expression in tumor cells, with non-neoplastic glands and stroma with normal expression pattern (original magnification ×100).
Immunohistochemical analysis of p53 and MMR proteins MLH1, MSH2, MSH6, and PMS2 (performed routinely at our institution) was repeated for this study on tissue microarray blocks, with the addition of L1CAM analysis. Tissue microarrays were constructed with four selected 2 mm-diameter tumor areas. Details of the immunohistochemical reactions are provided in Table 1. All reactions were performed using the Envision Flex detection system (Dako, Carpinteria, USA), and peroxidase activity was visualized with diaminobenzidine staining (DAKO, USA).
Table 1.
–Immunohistochemistry method details.
| Primary antibody | Clone | Provider | Titration | Antigen retrieval | Time of antigen retrieval |
|---|---|---|---|---|---|
| p53 | DO-7 | Dako | 1:3000 | pH 9.0 | 20 min |
| MLH1 | ES05 | Dako | Pure; Linker mouse | pH 9.0 | 20 min |
| MSH2 | G219-1129 | Dako | 1:400 | pH 9.0 | 30 min |
| MSH6 | EP49 | Dako | pure; Linker mouse | pH 9.0 | 20 min |
| PMS2 | EP51 | Dako | pure; Linker mouse | pH 9.0 | 40 min |
| L1CAM | 14.10 | COVANCE | 1:200 | pH 6.1 | 20 min |
MMRd was defined as the complete or subclonal abrupt loss of the nuclear expression of at least one of the proteins in tumor cells in the presence of internal control positivity in lymphocytes and/or stroma (Fig. 1B and C) [22]. For statistical purposes, we divided MMRd cases into two groups: one with MLH1/PMS2 loss and MSH2 and MSH6 preservation, and the other with MSH2 and/or MSH6 loss, independent of MLH1/PMS2 status. MMRp status was defined as the preservation of the immunohistochemical expression of all four proteins. p53mut status was defined by strong uniform nuclear staining in >80% of the tumor cells, or complete absence of such staining, in the presence of focal nuclear staining of the stromal cells (Fig. 1F). p53w status was defined by weak to moderate nuclear staining in some tumor cells (Fig. 1E). L1CAM positivity was defined as membrane staining in 10% or more tumor cells [23] (Fig. 1D).
The chi-square test and Fisher's exact test were used to evaluate the association between categorical variables. Patient age, BMI, and tumor size were treated as quantitative variables and compared using the Mann-Whitney U test, t-test), and Kruskal-Wallis test with Dunn adjustment for multiple group comparison. Kaplan–Meier curves were used to describe recurrence-free survival and/or death related to the disease, with the inclusion of data from patients with >3 months follow-up. Survival curves were compared using the log rank test. Stepwise logistic regression was used to select the variables associated with lymph node metastasis, and Cox proportional hazards regression was used to analyze the effect of multiple variables on outcomes.
The statistical analyses were performed using MedCalc® software (version 20.123; Medcalc Software Ltd., Ostend, Belgium; https://www.medcalc.org; 2022). P-values ≤0.05 were considered to be significant.
3. Results
In total, 312 consecutive patients aged 36–85 years (mean ± standard deviation, 63.1 ± 7.99) were included. Of these patients, 116 (37.2%) had MMRd and 196 (62.8%) had MMRp tumors. The median follow-up duration was 54.5 (range 0–120.5) months [95% confidence interval (CI), 51.7–56.8 months]. Adjuvant chemotherapy was indicated in 134 (42.9%) patients, of whom 132 (98.5%) received carboplatin and paclitaxel regimens and 2 (1.5%) received cisplatin and doxorubicin regimens. External radiotherapy was performed in 138 (44.2%) and brachytherapy in 190 (60.8%). Eighty-four of 291 (28.9%) patients had family histories of cancer [endometrial, n = 21 (7.2%); colorectal, n = 21 (7.2%); breast, n = 34 (11.7%); ovarian, n = 6 (2.1%); stomach, n = 1 (0.3%); thyroid, n = 1 (0.3%)].
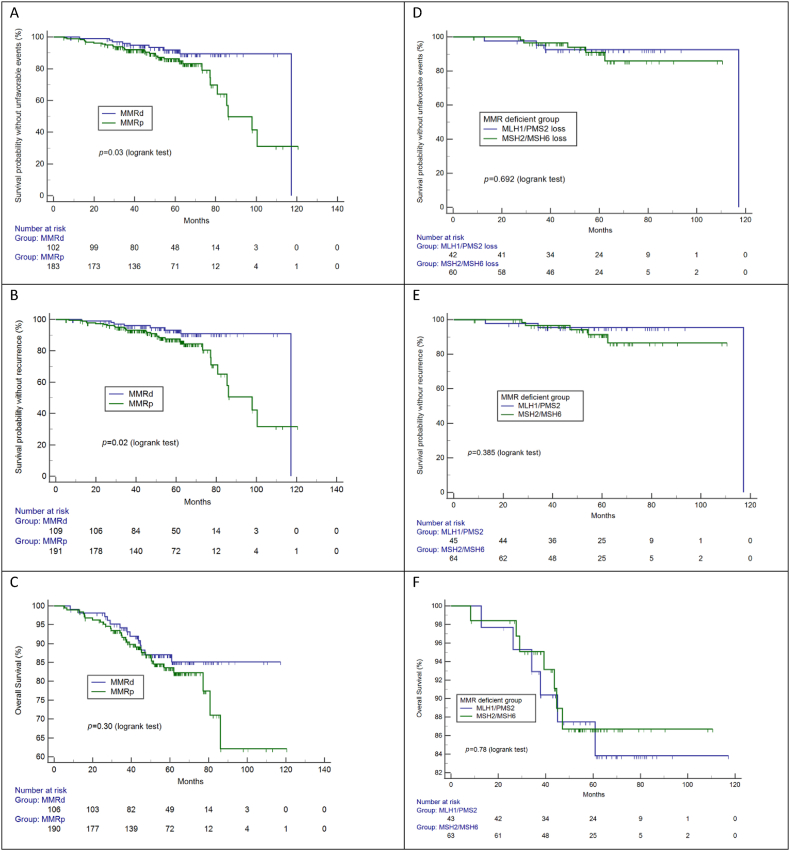
The clinicopathological findings are summarized in Table 2. More MMRd than MMRp tumors were associated with endometrioid histology (p = 0.008). MMRd and MMRp cases did not differ in terms of age, family history of cancer, family history of endometrial and/or colorectal cancer, BMI, tumor stage, histological grade, tumor size, depth of myometrial invasion, or pattern of myoinvasion. LVSI was more frequent in MMRd tumors than in MMRp tumors (p = 0.02), but lymph node metastasis was similar in the two groups. The distribution of ESMO/ESTRO/ESGO risk categories differed between groups, but not in a clinically relevant manner. Intermediate risk was more frequent in MMRp tumors, and high-intermediate risk was more prevalent among MMRd group. Twenty (17.2%) and 41 (20.9%) MMRd and MMRp tumors, respectively, were of intermediate or high-intermediate risk (p = 0.05). More relapse occurred in the MMRp group than in the MMRd group, but this difference did not affect disease-related survival. Survival curves according to MMR status are provided in Fig. 2 (A-F). Eight patients died in the immediate postoperative period due to complications not related directly to the disease, and their data were not included in the survival analyses.
Table 2.
Clinicopathological characteristics of 312 endometrial cancers according to DNA mismatch repair (MMR) protein status.
| MMR deficient | MMR proficient | p | ||
|---|---|---|---|---|
| n | 116 | 196 | ||
| age | Median (95%CI) | 63.2 (60.1–64.8) | 63.1 (61.9–63.8) | 0.98 |
| Family history of cancer | Yes | 38 (34.2%) | 46 (25.5%) | 0.26 |
| No | 73 (65.8%) | 134 (74.4%) | ||
| Body Mass Index | Median (95%CI) | 31.1 (29.4–32.4) | 30.4 (29.7–31.6) | 0.33 |
| Histological type | Endometrioid | 102 (87.9%) | 148 (75.5%) | 0.008 |
| Non-endometrioid | 14 (12.1%) | 48 (24.5%) | ||
| FIGO | I/II | 81 (69.8%) | 139 (70.9%) | 0,84 |
| III/IV | 35 (30.2%) | 57 (29.1%) | ||
| ESMO-ESGO-ESTRO | low | 38 (32.8%) | 59 (30.1%) | 0.020 |
| intermediate | 5 (4.3%) | 29 (14.8%) | ||
| High-intermediate | 15 (12.9%) | 12 (6.1%) | ||
| High | 54 (46.6%) | 91 (46.4%) | ||
| Advanced/metastatic | 4 (3.4%) | 5 (2.6%) | ||
| Grade | Low | 85 (73.3%) | 137 (69.9%) | 0,52 |
| High | 31 (26.7%) | 59 (30.1%) | ||
| Tumor size (cm) | Mean (95% CI) | 4 (3.7–4.5) | 4 (3.7–4.5) | 0.84 |
| Tumor size | ≤2.0 cm | 13 (12.4%) | 27 (15.3%) | 0,50 |
| >2.0 cm | 92 (87.6%) | 150 (84.7%) | ||
| NA | 11 | 19 | ||
| Myometrial invasion | <50% | 61 (52.6%) | 109 (55.9%) | 0,57 |
| ≥50% | 55 (47.4%) | 86 44.1%) | ||
| NA | 1 | |||
| LVSI | No/focal | 84 (72.4%) | 162 (83.1%) | 0.025 |
| Multifocal | 32 (27.6%) | 33 (16.9%) | ||
| NA | 1 | |||
| MELF | Absent | 89 (76,7%) | 132 (67.7%) | 0.090 |
| Present | 27 (23.3%) | 63 (32.3%) | ||
| NA | 0 | 1 | ||
| Node | Negative | 88 (77.2%) | 154 (61.8%) | 0,78 |
| Positive | 26 (22.8%) | 42 (21.4%) | ||
| NA | 2 | 0 | ||
| Recurrence | 8/115 (6.9%) | 29/195 (14.8%) | 0.02*** | |
| Death of disease | 9/110 (8.2%) | 22/192 (11.5%) | 0.31*** | |
| Any unfavorable event (recurrence and/or death associated with disease) | 11/106 (10.38%) | 32/185 (17.5%) | 0.07*** |
LVSI: lymphovascular space invasion; MELF: microcystic, elongated, and fragmented; NA: not avaible.
*Mann-Whitney test; ** Chi-square test; *** logrank test.
Fig. 2.
Survival outcomes according DNA mismatch repair (MMR) protein status. A, B and C: MMR-deficient vs. -proficient tumors; D, E and F: MLH1/PMS2 loss vs. MSH2/MSH6 loss.
Fifty MMRd tumors showed MLH1/PMS2 loss, 51 had MSH2/MSH6 loss, and 15 of these tumors showed both types of loss (Table 3). Patients with MSH2/MSH6 loss tended to be younger than those with MLH1/PMS2 loss, but this difference was not significant. A family history of cancer was more frequent among patients with MSH2/MSH6 loss than among those with MLH1/PMS2 loss (p = 0.05) and MMRp group (p = 0.007). This difference was maintained when we compared the frequencies of colorectal and/or endometrial cancer between MSH2/MSH6 loss and MLH1/PMS2 loss (p = 0.04) and between MSH2/MSH6 loss and MMRp (p = 0.03). More tumors in the MSH2/MSH6 loss group than in the MLH1/PMS2 loss group were early stage (p = 0.005), with lower ESMO/ESTRO/ESGO risk profiles (p = 0.007), lower tumor size (p = 0.01), less ≥50% myometrial invasion (p = 0.006), less LVSI (p = 0.03), and less lymph node metastasis (p = 0.01). However, outcomes did not differ between groups.
Table 3.
Clinicopathological characteristics of the two types of DNA mismatch repair-deficient (MLH1/PMS2 and MSH2/MSH6 losses) and -proficient endometrial carcinomas.
| Variable | MLH1/PMS2 (1) | MSH2/MSH6 (2) | MMRp (3) | P (1 vs. 2) | P (1 vs 3) | P (2 vs. 3) |
|---|---|---|---|---|---|---|
| n | 50 | 66 | 196 | |||
| Age (y); median (95% CI) | 64.1 (60.67–65.56) | 61.2 (59.22–65.52) | 63.1 (61.9–63.8) | 0.62* | 0.73* | 0,74* |
| Family history of cancer | ||||||
| no | 37 (78.7%) | 36 (56.2%) | 134 (74.4%) | 0.05** | 0.54** | 0.007** |
| yes | 10 (21.2%) | 28 (40.7%) | 46 (25.5%) | |||
| Body Mass Index; median (95%CI) | 31.2 (29.5–32.6) | 31 (28.8–33.8) | 30.4 (29.7–31.6) | 0.46* | 0.34* | 0.05* |
| Histological type: | ||||||
| Endometrioid | 45 (90%) | 57 (86.4%) | 148 (75.5%) | 0.55** | 0.03** | 0.06** |
| Non-endometrioid | 5 (10%) | 9 (13.6) | 48 (24.5%) | |||
| FIGO stage | ||||||
| I/II | 28 (56%) | 53 (80.3%) | 139 (70.9%) | 0.005** | 0.04** | 0.14** |
| III/IV | 22 (44%) | 13 (19.7%) | 57 (29.1%) | |||
| ESMO-ESGO-ESTRO risk stratification | ||||||
| low | 10 (20%) | 28 (42.4%) | 59 (30.1%) | 0.007** | 0.14** | 0.02** |
| intermediate | 2 (4%) | 3 (4.5%) | 29 (14.8%) | |||
| High-intermediate | 6 (12%) | 9 (13.6%) | 12 (6.1%) | |||
| High | 20 (60%) | 24 (36.4%) | 91 (46.4%) | |||
| Advanced/metastatic | 2 (4%) | 2 (3%) | 5 (2.6%) | |||
| Low tumor grade | 36 (72%) | 49 (74.2%) | 137 (69.9%) | 0.78** | 0.50** | 0.77** |
| High tumor grade | 14 (28%) | 17 (25.8%) | 59 (30.1%) | |||
| Tumor size (cm); median (95% CI) | 4.5 (4–5.2) | 3.9 (3.4–4.4) | 4 (3.7–4.5) | 0.01* | 0.06* | 0.20* |
| Tumor size | ||||||
| ≤2.0 cm | 5 (11.1%) | 8 (13.3%) | 27 (15.3%) | 0.73** | 0.48** | 0.71** |
| >2.0 cm | 40 (88.9%) | 52 (86.7%) | 150 (84.7%) | |||
| Not evaluated | 5 | 6 | 19 | |||
| Myometrial invasion | ||||||
| <50% | 19 (38%) | 42 (63.6%) | 109 (55.9%) | 0.006** | 0.02** | 0.27** |
| ≥50% | 31 (62%) | 24 (36.4%) | 86 (44.1%) | |||
| LVSI | ||||||
| No/focal | 31 (62%) | 53 (80.3%) | 162 (83.1%) | 0.03** | 0.001 | 0.61** |
| Multifocal | 19 (38%) | 13 (19.7%) | 33 (16.9%) | |||
| MELF | ||||||
| Absent | 36 (72%) | 53 (80.3%) | 132 (67.7%) | 0.30** | 0.56** | 0.05** |
| Present | 14 (28%) | 13 (19.7%) | 63 (32.3%) | |||
| Node | ||||||
| Negative | 33 (66%) | 55 (85.9%) | 154 (61.8%) | 0.012** | 0.06** | 0.20** |
| Positive | 17 (34%) | 9 (14.1%) | 42 (21.4%) | |||
| Not evaluated | 0 | |||||
LVSI: lymphovascular space invasion; MELF: microcystic, elongated, and fragmented.
*t-test; **Chi-square test, for p-values analyzing the three groups together, see text.
Age, tumor grade, and BMI did not differ between the MMRd tumor subgroups and the MMRp subgroup. Endometrioid histology was more common in the MLH1/PMS2 (p = 0.03) and MSH2/MSH6 loss groups (p = 0.06) than in the MMRp group. Relative to MMRp tumors, more tumors with MSH2/MSH6 loss had low ESMO-ESGO-ESTRO risk profile (p = 0.02) and more tumors with MLH1/PMS2 loss were advanced (p = 0.04), with deeper myometrial infiltration (p = 0,02), more LVSI (p = 0.001), and more lymph node metastasis (p = 0.06) (Table 3). The median tumor size was larger in the MLH1/PMS2 loss group than in the MMRp group, but this difference was not significant in the adjusted analysis. MMRp tumors were associated with more recurrence than were the MMRd subgroups, but this difference had no impact on survival (Table 4).
Table 4.
Relative risk of lymph node metastasis and outcome according MMR status, p53 expression and L1CAM expression in 312 endometrial carcinomas.
| Lymph node metastasis |
recurrence |
death |
Recurrence and/or death |
|
|---|---|---|---|---|
| RR (95% CI, p) | RR (95% CI, p) | RR (95% CI, p) | RR (95% CI, p) | |
| MMRd | 1.06 (0.69–1.64, 0.78) | 0.47 (0.22–0.99, 0.05) | 1.02 (0.61–1.71, 0.94) | 0.60 (0.32–1.14, 0.12) |
| p53mut | 3.06 (2.08–4.50, <0.0001) | 5.26 (2.96–9.37, <0.0001) | 4.24 (2.24–8.04, <0.0001) | 3.85 (2.29–6.46, <0.0001) |
| L1CAM-positive | 2.93 (1.97–4.34, <0.0001) | 4.58 (2.53–8.29, <0.0001) | 2.43 (1.48–3.96, 0.0004) | 3.57 (2.10–6.07, <0.0001) |
p53mut immunoexpression was identified in 56 cases, 12 (21.4%) in MMRd tumors and 44 (78.6%) in MMRp tumors. These cases represented 10.6% of MMRd tumors and 23.2% of MMRp tumors (p = 0.007). Among MMRd tumors, 10 (17.8%) of these cases had MSH2/MSH6 loss and 2 (3.5%) had MLH1/PMS2 loss (p = 0.06). L1CAM positivity was observed in 69 [22.1%; 13 (18.8%) MMRd and 56 (81.2%) MMRp] tumors. These cases represented 11.2% of MMRd tumors and 28.6% of MMRp tumors (p = 0.0004). L1CAM positivity did not differ significantly between the MLH1/PMS2 and MSH2/MSH6 loss subgroups (8% and 13.6%, respectively). The p53mut status was associated strongly with L1CAM positivity: 219 (72.3%) tumors were L1CAM negative/p53w, 41 (13.5%) were L1CAM positive/p53mut, 15 (5%) were L1CAM positive/p53w, and 28 (9.2%) tumors were L1CAM negative/p53mut (p < 0.0001).
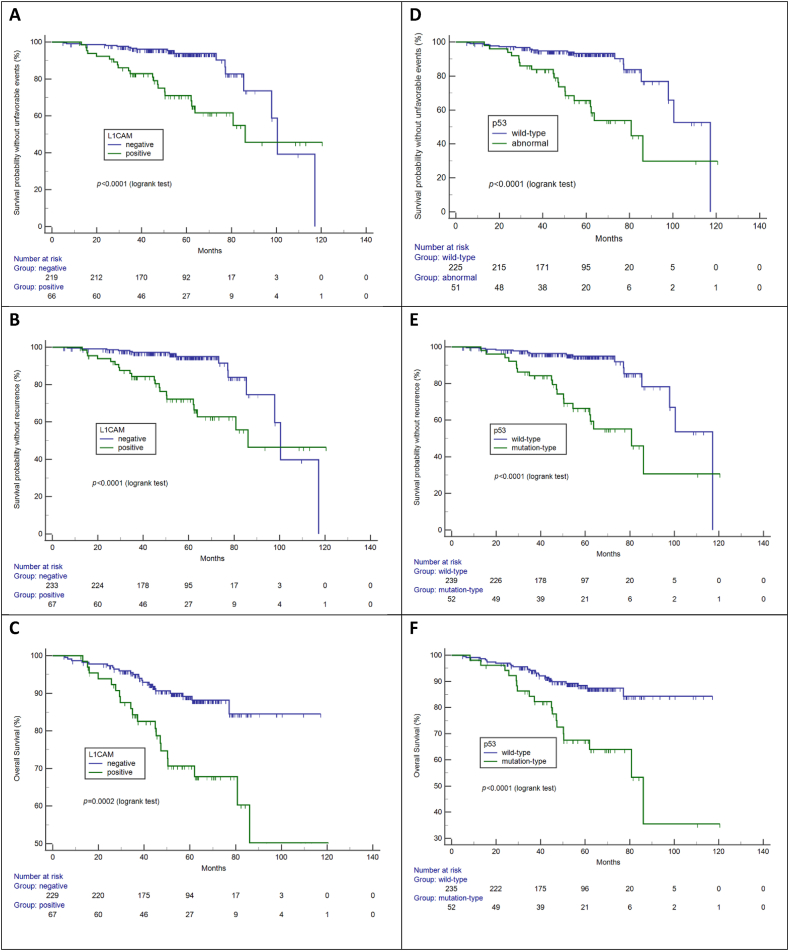
L1CAM and p53 expression were associated with a greater risk of lymph node metastasis and higher rates of recurrence and death (Table 4). Curves of survival according to L1CAM (Fig. 3A–C) and p53 (Fig. 3D–F) expression demonstrate the prognostic value of these variables.
Fig. 3.
Survival outcomes according L1CAM (A, B, C) and p53 (D, E, F).
The predictive ability of the following variables with regard to lymph node metastasis was examined in a multivariate model: tumor size, histological type, tumor grade, LVSI, depth of myometrial invasion, MMR protein status, p53 status, and L1CAM status. Only tumor size, non-endometrioid histology, and multifocal LVSI were predictive (Table 5). For endometrioid tumors, only tumor size [odd ratio (OR) 1.27; 95%CI 1.09–1.47; p = 0.001] and LVSI (OR 3.71; 95% CI 1.55–8.86; p = 0.003) were predictive (p < 0.0001). For MMRd group, only tumor size (OR 1.2; 95% CI 1.02–1.50; p = 0.03) and >50% myometrial invasion (OR 3.3; 95% CI 1.11–10.10; p = 0.03) were predictive of lymph node metastasis (p = 0.0005).
Table 5.
Independent variables associated with lymph node involvement in 312 endometrial carcinomas.
| Variable | OR | 95%CI | p |
|---|---|---|---|
| All cohort | |||
| Tumor size | 1.35 | 1.18–1.55 | <0.0001 |
| Multifocal LVSI | 5.18 | 2.50–10.70 | <0.0001 |
| Non-endometrioid histology | 3.83 | 1.81–8.11 | 0.0005 |
| Endometrioid carcinomas | |||
| Tumor size | 1.27 | 1.10–1.48 | 0.0013 |
| Multifocal LVSI | 3.89 | 1.63–9.30 | 0.0022 |
LVSI: lymphovascular space invasion.
A Cox proportional-hazards regression model was created with the variables FIGO stage, histological type, grade, tumor size, depth of myometrial invasion, LVSI, lymph node metastasis, MMR protein status, p53 status, and L1CAM status. Factors predictive of unfavorable events (recurrence and/or death) were non-endometrioid histology type (p < 0.0001), advanced stage (p = 0.0224), and deep myometrial invasion (p = 0.0053). For endometrioid carcinomas, only advanced stage was predictive (p = 0.03). Factors associated with recurrence were non-endometrioid histology (p < 0.0001) and deep myometrial infiltration (p = 0.0004). For endometrioid tumors, only LVSI was predictive of recurrence (p = 0.0445).
4. Discussion
In the present study, the clinicopathological features and outcomes of endometrial carcinoma were analyzed, with an emphasis on MMR proteins status. The study groups did not differ in terms of age, BMI, FIGO stage, tumor grade, tumor size, depth of myometrial infiltration, or lymph node metastasis. The only differences observed were that endometrioid histology and LVSI were more common in MMRd than in MMRp tumors.
The association of MMRd status with endometrioid histology is well documented [24,25], but the frequency of non-endometrioid MMRd tumors (22.6% in our sample, 13.8% in Kim et al. [25]) is not negligible. Considering the role of immune checkpoint inhibitors as a therapeutic option, the determination of the MMR protein status in all cases, regardless of histological characteristics, is important.
LVSI, a classical prognostic factor, has also been observed more frequently among MMRd tumors in previous studies [[24], [25], [26]]. However, the prognostic value of this indicator is controversial and likely depends on other factors, such as immune activation, which is more common in tumors with chromatin dysregulation [27,28]. In addition to its association with the MMRd status, LVSI was associated with endometrioid tumor recurrence in our sample.
The MMRd status has been associated with adverse outcomes in some studies [24,26], but found to be favorable for survival in other work [29]. We observed less recurrence of MMRd group than of MMRp tumors in the present study. The MMR protein status is also related to the treatment response [25,29]. Possible explanations for biological differences associated with MMR status include geographical or ethnic influence, immune activation, and the origin (epigenetic, germinative or somatic) and/or type of the protein loss. Most cases of MLH1/PMS2 loss are secondary to MLH1 methylation, whereas other types of MMR protein loss are more commonly associated with Lynch syndrome [26,29]. Many patients in our MSH2/MSH6 loss group had family histories of cancer, suggesting that Lynch syndrome was more common in this group. We do not have access to testing for this syndrome for all patients at our public institution. Relative to MMRd tumors with MLH1/PMS2 loss, tumors in our sample with MSH2/MSH6 loss were smaller and have less depth of myoinvasion, LVSI and lymph node involvement. MSH2/MSH6 loss presented with smaller tumors, less ≥50% myoinvasion, less LVSI, and less lymph node involvement than those with MLH1/PMS2 loss. However, recurrence and death rates did not differ between these groups, suggesting that other factors are involved in the more favorable outcomes observed in these subgroups than in the MMRp group. One factor to consider is immune activation, present in MMRd tumors and potentially indicating immunotherapy [14,27,30]. MLH1 methylation corresponds to more than 70% of MLH1 deficiency and more aggressive clinicopathological features [11,31].
Some of our cases of MSH2/MSH6 loss included some less usual patterns of staining, characterized by loss of protein for both heterodimers. Although this finding has been described, mainly for tumors of the digestive system, its biological significance is not understood [32,33]. As MMR protein testing is increasingly included in the diagnostic workup for endometrial carcinoma, based on the recommendations of numerous medical societies [6,[34], [35], [36]], an increasing number of cases may show this pattern, highlighting the need to recognize and further investigate it. Overall, the characterization of all patterns of MMR protein loss, including subclonal loss, heterodimer involvement, and anomalous expression patterns (e.g., punctate nuclear [37]), is very important. Even subclonal staining, for example, has been found associated to LS [38]. In addition to serving as a screening tool for Lynch syndrome, MMR protein testing provides information that is useful for the selection of systemic therapy (i.e., indication for immune checkpoint inhibitors alone or in combination with chemotherapy) for some conditions [17,39,40]. About 30% of MMRd cases in our sample were advanced and about 50% were classified as high/advanced risk (∼50%); immunotherapy is an option for such cases.
Strong L1CAM expression has been associated with adverse clinicopathological factors and worse prognoses of endometrial carcinomas, particularly those with nonspecific molecular profiles [41,42]. In our previous study, in a small sample, L1CAM expression was associated with adverse clinicopathological features, particularly LVSI [43]. p53mut status has been noted to be an important prognostic factor [44]. The present study confirmed the prognostic role of L1CAM and p53mut status, but these features were not independent in the multivariate analysis. L1CAM was associated with p53mut, and both were less common among MMRd tumors. TP53 mutation was described in 5% of MSI-H tumors in the TCGA study [3]. Among cases of multiple molecular classifiers, MMRd/p53mut is the most common profile, present in about 1.8% of the cases, and does not seem to impact the behavior of MMRd tumors [45]. In our sample, 10.6% of MMRd tumors had p53mut status; this proportion is smaller than that of MMRp tumors (23.2%), but larger than in the TCGA dataset. One possible explanation for this difference is that some of these cases are POLE-mutated tumors, 35% of which have TP53 mutations according to TCGA [3].
In our multivariate analysis, tumor size was an independent prognostic factor for lymph node metastasis in the entire cohort and for MMRd tumors. Although most of these cases were early stage, small proportion of tumors with diameters <2.0 cm was notable, reflecting the difficulty of early diagnosis. In our previous study of 703 patients treated at the same institution from 2008 to 2018, the same low frequency of tumors smaller than 2.0 cm was observed (i.e., 13.7 cm A similarly small proportion of endometrial tumors with diameters <2.0 cm was observed in a sample of 703 patients treated at the same institution from 2008 to 2018 (mean tumor diameter, 13.7 cm) [46]. This cut-off is critical for the application of more conservative surgical approaches and has been shown to have a prognostic impact [[47], [48], [49]].
The present study has several strengths. First, it was conducted with an unselected cohort of patients diagnosed and treated at a single reference cancer center by a uniform pathological, surgical, and oncological team whose members follow rigid guidelines. In addition, the same senior pathologist reviewed all cases. Second, the study explored MMRd tumors, which are common among endometrial carcinomas and should be identified for the purposes of Lynch syndrome screening and therapeutic guidance. The present results suggest that differences between MMRd types should be further investigated to better understand their possible causes and clinical implications. Finally, although the study was retrospective, it provides a real-life picture of endometrial cancer, the incidence of which is increasing. A study limitation was the impossibility of examining MLH1 hypermethylation and Lynch syndrome due to the lack of avaible testing. Another limitation was the lack of POLE sequencing, which is not performed widely in Brazil, especially in public hospitals.
5. Conclusion
MMRd endometrial carcinomas are frequent and more commonly of endometrioid histology and associated with less recurrence than MMRp tumors, although these factors have no impact on survival. L1CAM positivity and p53mut status were more common in MMRp tumors than in MMRd tumors in our sample and were associated with worse outcomes, although they were not independent factors. Relative to MLH1/PMS2 loss, MSH2/MSH6 loss in MMRd tumors was associated with a family history of cancer, earlier diagnosis, smaller tumor size, and less myometrial invasion, LVSI and lymph node metastasis. However, the recurrence and disease-specific death rates associated with these two MMRd tumor types were similar. The observed differences may be related to the greater probability of Lynch syndrome associated with MSH2/MSH6 loss relative to MLH1/PMS2 loss.
MMRd tumors present in advanced stages and with high-risk features in a fraction similar to proficient tumors. Similar proportions of MMRd and MMRp tumors present in advanced stages and with high-risk features. The precise identification of MMR protein status is crucial to determine patient candidacy for single-agent immunotherapy.
Funding
This study was supported by São Paulo Research Foundation (FAPESP) (process #2018/06717-8).
Author contribution statement
Filomena Marino Carvalho: conceived and designed the experiments; analyzed and interpreted the data; contributed reagents, materials, analysis tools or data; wrote the paper. Daniela de Freitas: analyzed and interpreted the data; wrote the paper. Fernando Nalesso Aguiar: performed the experiments; Cristina Anton: analyzed and interpreted the data; Danielle Cristina de Almeida: performed the experiments; Carlos Eduardo Bacchi: performed the experiments; contributed reagents, materials, analysis tools or data. Jesus Paula Carvalho: conceived and designed the experiments; wrote the paper.
Data availability statement
Data will be made available on request.
Additional information
No additional information is available for this paper.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2022. Ca - Cancer J. Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Estatísticas de câncer - Instituo Nacional de Cancer - I.N.C.A. 2022. https://www.gov.br/inca/pt-br/assuntos/cancer/numeros
- 3.Kandoth C., Schultz N., Cherniack A.D., Akbani R., Liu Y., Shen H., Robertson A.G., Pashtan I., Shen R., Benz C.C., et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Talhouk A., McConechy M.K., Leung S., Yang W., Lum A., Senz J., Boyd N., Pike J., Anglesio M., Kwon J.S., et al. Confirmation of ProMisE: a simple, genomics-based clinical classifier for endometrial cancer. Cancer. 2017;123:802–813. doi: 10.1002/cncr.30496. [DOI] [PubMed] [Google Scholar]
- 5.Colombo N., Creutzberg C., Amant F., Bosse T., González-Martín A., Ledermann J., Marth C., Nout R., Querleu D., Mirza M.R., et al. ESMO-ESGO-ESTRO consensus conference on endometrial cancer: diagnosis, treatment and follow-up. Int. J. Gynecol. Cancer. 2016;26:2–30. doi: 10.1097/IGC.0000000000000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oaknin A., Bosse T.J., Creutzberg C.L., Giornelli G., Harter P., Joly F., Lorusso D., Marth C., Makker V., Mirza M.R., et al. Endometrial cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022;33:860–877. doi: 10.1016/j.annonc.2022.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Hua T., Liu S., Xin X., Jin Z., Liu Q., Chi S., Wang X., Wang H. Prognostic significance of L1 cell adhesion molecule in cancer patients: a systematic review and meta-analysis. Oncotarget. 2016;7:85196–85207. doi: 10.18632/oncotarget.13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang J.W., Wang S.Q., Wu Z.Y., Liu Q., Yuan Q., Cai H.Q., Wan J.H. L1 cell adhesion molecule high expression is associated with poor prognosis in surgically resected brain metastases from lung adenocarcinoma. Clinics. 2022;77 doi: 10.1016/j.clinsp.2022.100040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mancusi de Carvalho J.P., Salim R.C., Carvalho F.M., Nogueira Dias Genta M.L., Baracat E.C., Carvalho J.P. L1 cell adhesion molecule (L1CAM) in stage IB cervical cancer: distinct expression in squamous cell carcinomas and adenocarcinomas. J. Clin. Pathol. 2020 doi: 10.1136/jclinpath-2020-206500. [DOI] [PubMed] [Google Scholar]
- 10.Bohaumilitzky L., von Knebel Doeberitz M., Kloor M., Ahadova A. Implications of hereditary origin on the immune phenotype of mismatch repair-deficient cancers: systematic literature review. J. Clin. Med. 2020;9 doi: 10.3390/jcm9061741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryan N.A.J., McMahon R., Tobi S., Snowsill T., Esquibel S., Wallace A.J., Bunstone S., Bowers N., Mosneag I.E., Kitson S.J., et al. The proportion of endometrial tumours associated with Lynch syndrome (PETALS): a prospective cross-sectional study. PLoS Med. 2020;17 doi: 10.1371/journal.pmed.1003263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.León-Castillo A., de Boer S.M., Powell M.E., Mileshkin L.R., Mackay H.J., Leary A., Nijman H.W., Singh N., Pollock P.M., Bessette P., et al. Molecular classification of the PORTEC-3 trial for high-risk endometrial cancer: impact on prognosis and benefit from adjuvant therapy. J. Clin. Oncol. 2020;38:3388–3397. doi: 10.1200/JCO.20.00549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russo A.L., Lee L.J., Wo J.Y., Niemierko A., Park D., Alban G., King M., Philp L., Growdon W.B., Oliva E., et al. Effect of mismatch repair status on outcome of early-stage grade 1 to 2 endometrial cancer treated with vaginal brachytherapy. Am. J. Clin. Oncol. 2022;45:36–39. doi: 10.1097/COC.0000000000000871. [DOI] [PubMed] [Google Scholar]
- 14.Di Dio C., Bogani G., Di Donato V., Cuccu I., Muzii L., Musacchio L., Scambia G., Lorusso D. The role of immunotherapy in advanced and recurrent MMR deficient and proficient endometrial carcinoma. Gynecol. Oncol. 2022;169:27–33. doi: 10.1016/j.ygyno.2022.11.031. [DOI] [PubMed] [Google Scholar]
- 15.Manning-Geist B.L., Liu Y.L., Devereaux K.A., Paula A.D.C., Zhou Q.C., Ma W., Selenica P., Ceyhan-Birsoy O., Moukarzel L.A., Hoang T., et al. Microsatellite instability-high endometrial cancers with MLH1 promoter hypermethylation have distinct molecular and clinical profiles. Clin. Cancer Res. 2022;28:4302–4311. doi: 10.1158/1078-0432.CCR-22-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaneko E., Sato N., Sugawara T., Noto A., Takahashi K., Makino K., Terada Y. Promoter hypermethylation predicts poorer prognosis in mismatch repair deficiency endometrial carcinomas. J Gynecol Oncol. 2021;32:e79. doi: 10.3802/jgo.2021.32.e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oaknin A., Gilbert L., Tinker A.V., Brown J., Mathews C., Press J., Sabatier R., O'Malley D.M., Samouelian V., Boni V., et al. Safety and antitumor activity of dostarlimab in patients with advanced or recurrent DNA mismatch repair deficient/microsatellite instability-high (dMMR/MSI-H) or proficient/stable (MMRp/MSS) endometrial cancer: interim results from GARNET-a phase I, single-arm study. J Immunother Cancer. 2022;10 doi: 10.1136/jitc-2021-003777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Malley D.M., Bariani G.M., Cassier P.A., Marabelle A., Hansen A.R., De Jesus Acosta A., Miller W.H., Safra T., Italiano A., Mileshkin L., et al. Pembrolizumab in patients with microsatellite instability-high advanced endometrial cancer: results from the KEYNOTE-158 study. J. Clin. Oncol. 2022;40:752–761. doi: 10.1200/JCO.21.01874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO Classification of Tumours Editorial Board . fifth ed. International Agency for Research on Cancer); 2020. Female Genital Tumours. [Google Scholar]
- 20.Mateva S., Nikolova M., Yordanov A. Patterns of myometrial invasion in endometrial adenocarcinoma with emphasizing on microcystic, elongated and fragmented (MELF) glands pattern: a narrative review of the literature. Diagnostics. 2021;11 doi: 10.3390/diagnostics11091707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peters E.E.M., León-Castillo A., Smit V.T.H.B., Boennelycke M., Hogdall E., Hogdall C., Creutzberg C., Jürgenliemk-Schulz I.M., Jobsen J.J., Mens J.W.M., et al. Defining substantial lymphovascular space invasion in endometrial cancer. Int. J. Gynecol. Pathol. 2022;41:220–226. doi: 10.1097/PGP.0000000000000806. [DOI] [PubMed] [Google Scholar]
- 22.Watkins J.C., Nucci M.R., Ritterhouse L.L., Howitt B.E., Sholl L.M. Unusual mismatch repair immunohistochemical patterns in endometrial carcinoma. Am. J. Surg. Pathol. 2016;40:909–916. doi: 10.1097/PAS.0000000000000663. [DOI] [PubMed] [Google Scholar]
- 23.Zeimet A.G., Reimer D., Huszar M., Winterhoff B., Puistola U., Azim S.A., Müller-Holzner E., Ben-Arie A., van Kempen L.C., Petru E., et al. L1CAM in early-stage type I endometrial cancer: results of a large multicenter evaluation. J. Natl. Cancer Inst. 2013;105:1142–1150. doi: 10.1093/jnci/djt144. [DOI] [PubMed] [Google Scholar]
- 24.Jumaah A.S., Al-Haddad H.S., Salem M.M., McAllister K.A., Yasseen A.A. Mismatch repair deficiency and clinicopathological characteristics in endometrial carcinoma: a systematic review and meta-analysis. J Pathol Transl Med. 2021;55:202–211. doi: 10.4132/jptm.2021.02.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim S.R., Pina A., Albert A., McAlpine J., Wolber R., Blake Gilks C., Kwon J.S. Does MMR status in endometrial cancer influence response to adjuvant therapy? Gynecol. Oncol. 2018;151:76–81. doi: 10.1016/j.ygyno.2018.08.020. [DOI] [PubMed] [Google Scholar]
- 26.Carr C., Son J., Yao M., Priyadarshini A., Marquard J., Vargas R., Michener C., AlHilli M.M. Clinicopathologic characteristics and outcomes of endometrial Cancer patients with mismatch repair deficiency in the era of universal Lynch syndrome screening. Gynecol. Oncol. 2020;159:712–720. doi: 10.1016/j.ygyno.2020.09.039. [DOI] [PubMed] [Google Scholar]
- 27.Guo Y.E., Liu Y., Zhang W., Luo H., Shu P., Chen G., Li Y. The clinicopathological characteristics, prognosis and immune microenvironment mapping in MSI-H/MMR-D endometrial carcinomas. Discov Oncol. 2022;13:12. doi: 10.1007/s12672-022-00466-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu Q., Tian R., Liu J., Ou C., Li Y., Fu X. Deciphering comprehensive features of tumor microenvironment controlled by chromatin regulators to predict prognosis and guide therapies in uterine corpus endometrial carcinoma. Front. Immunol. 2023;14 doi: 10.3389/fimmu.2023.1139126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shikama A., Minaguchi T., Matsumoto K., Akiyama-Abe A., Nakamura Y., Michikami H., Nakao S., Sakurai M., Ochi H., Onuki M., et al. Clinicopathologic implications of DNA mismatch repair status in endometrial carcinomas. Gynecol. Oncol. 2016;140:226–233. doi: 10.1016/j.ygyno.2015.11.032. [DOI] [PubMed] [Google Scholar]
- 30.Dong D., Lei H., Liu D., Bai H., Yang Y., Tang B., Li K., Liu J., Xu G., Xiao X. POLE and mismatch repair status, checkpoint proteins and tumor-infiltrating lymphocytes in combination, and tumor differentiation: identify endometrial cancers for immunotherapy. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.640018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pasanen A., Loukovaara M., Bützow R. Clinicopathological significance of deficient DNA mismatch repair and MLH1 promoter methylation in endometrioid endometrial carcinoma. Mod. Pathol. 2020;33:1443–1452. doi: 10.1038/s41379-020-0501-8. [DOI] [PubMed] [Google Scholar]
- 32.Moreno E., Rosa-Rosa J.M., Caniego-Casas T., Ruz-Caracuel I., Perna C., Guillén C., Palacios J. Next generation sequencing to decipher concurrent loss of PMS2 and MSH6 in colorectal cancer. Diagn. Pathol. 2020;15:84. doi: 10.1186/s13000-020-01001-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reitsam N.G., Märkl B., Dintner S., Waidhauser J., Vlasenko D., Grosser B. Concurrent loss of MLH1, PMS2 and MSH6 immunoexpression in digestive system cancers indicating a widespread dysregulation in DNA repair processes. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.1019798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Concin N., Matias-Guiu X., Vergote I., Cibula D., Mirza M.R., Marnitz S., Ledermann J., Bosse T., Chargari C., Fagotti A., et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer. 2021;31:12–39. doi: 10.1136/ijgc-2020-002230. [DOI] [PubMed] [Google Scholar]
- 35.NCCN Guidelines - Endometrial Carcinoma. 2023. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1473 [Google Scholar]
- 36.Matias-Guiu X., Selinger C.I., Anderson L., Buza N., Ellenson L.H., Fadare O., Ganesan R., Ip P.P.C., Palacios J., Parra-Herran C., et al. Data set for the reporting of endometrial cancer: recommendations from the international collaboration on cancer reporting (ICCR) Int. J. Gynecol. Pathol. 2022;41:S90–S118. doi: 10.1097/PGP.0000000000000901. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Q., Young G.Q., Yang Z. Pure discrete punctate nuclear staining pattern for MLH1 protein does not represent intact nuclear expression. Int. J. Surg. Pathol. 2020;28:146–152. doi: 10.1177/1066896919878830. [DOI] [PubMed] [Google Scholar]
- 38.Scheiderer A., Riedinger C., Kimball K., Kilgore L., Orucevic A. Reporting subclonal immunohistochemical staining of mismatch repair proteins in endometrial carcinoma in the times of ever-changing guidelines. Arch. Pathol. Lab Med. 2022;146:1114–1121. doi: 10.5858/arpa.2021-0201-OA. [DOI] [PubMed] [Google Scholar]
- 39.Hill B.L., Graf R.P., Shah K., Danziger N., Lin D.I., Quintanilha J., Li G., Haberberger J., Ross J.S., Santin A.D., et al. Mismatch repair deficiency, next-generation sequencing-based microsatellite instability, and tumor mutational burden as predictive biomarkers for immune checkpoint inhibitor effectiveness in frontline treatment of advanced stage endometrial cancer. Int. J. Gynecol. Cancer. 2023 doi: 10.1136/ijgc-2022-004026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Makker V., Aghajanian C., Cohn A.L., Romeo M., Bratos R., Brose M.S., Messing M., Dutta L., Dutcus C.E., Huang J., et al. A phase ib/II study of lenvatinib and pembrolizumab in advanced endometrial carcinoma (study 111/KEYNOTE-146): long-term efficacy and safety update. J. Clin. Oncol. 2023;41:974–979. doi: 10.1200/JCO.22.01021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo M., Gong H., Nie D., Li Z. High L1CAM expression predicts poor prognosis of patients with endometrial cancer: a systematic review and meta-analysis. Medicine (Baltim.) 2021;100 doi: 10.1097/MD.0000000000025330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kommoss F.K., Karnezis A.N., Kommoss F., Talhouk A., Taran F.A., Staebler A., Gilks C.B., Huntsman D.G., Krämer B., Brucker S.Y., et al. L1CAM further stratifies endometrial carcinoma patients with no specific molecular risk profile. Br. J. Cancer. 2018;119:480–486. doi: 10.1038/s41416-018-0187-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Freitas D., Aguiar F.N., Anton C., Bacchi C.E., Carvalho J.P., Carvalho F.M. L1 Cell Adhesion Molecule (L1CAM) expression in endometrioid endometrial carcinomas: a possible pre-operative surrogate of lymph vascular space invasion. PLoS One. 2018;13 doi: 10.1371/journal.pone.0209294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yano M., Ito K., Yabuno A., Ogane N., Katoh T., Miyazawa M., Hasegawa K., Narahara H., Yasuda M. Impact of TP53 immunohistochemistry on the histological grading system for endometrial endometrioid carcinoma. Mod. Pathol. 2019;32:1023–1031. doi: 10.1038/s41379-019-0220-1. [DOI] [PubMed] [Google Scholar]
- 45.León-Castillo A., Gilvazquez E., Nout R., Smit V.T., McAlpine J.N., McConechy M., Kommoss S., Brucker S.Y., Carlson J.W., Epstein E., et al. Clinicopathological and molecular characterisation of 'multiple-classifier' endometrial carcinomas. J. Pathol. 2020;250:312–322. doi: 10.1002/path.5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anton C., Kleine R.T., Mayerhoff E., Diz M.D.P.E., Freitas D., Carvalho H.A., Carvalho J.P.M., Silva A.S.E., Genta M.L.N.D., Silva A.L.F.E., et al. Ten years of experience with endometrial cancer treatment in a single Brazilian institution: patient characteristics and outcomes. PLoS One. 2020;15 doi: 10.1371/journal.pone.0229543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.AlHilli M.M., Podratz K.C., Dowdy S.C., Bakkum-Gamez J.N., Weaver A.L., McGree M.E., Keeney G.L., Cliby W.A., Mariani A. Risk-scoring system for the individualized prediction of lymphatic dissemination in patients with endometrioid endometrial cancer. Gynecol. Oncol. 2013;131:103–108. doi: 10.1016/j.ygyno.2013.06.037. [DOI] [PubMed] [Google Scholar]
- 48.Jin X., Shen C., Yang X., Yu Y., Wang J., Che X. Association of tumor size with myometrial invasion, lymphovascular space invasion, lymph node metastasis, and recurrence in endometrial cancer: a meta-analysis of 40 studies with 53,276 patients. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.881850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matsushita C., Fujiwara H., Takei Y., Saga Y., Machida S., Taneichi A., Takahashi S., Yoshiba T., Koyanagi T., Takahashi Y., et al. New criteria for the omission of lymphadenectomy in endometrioid carcinoma. Int. J. Gynecol. Cancer. 2019;29:541–546. doi: 10.1136/ijgc-2018-000044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.