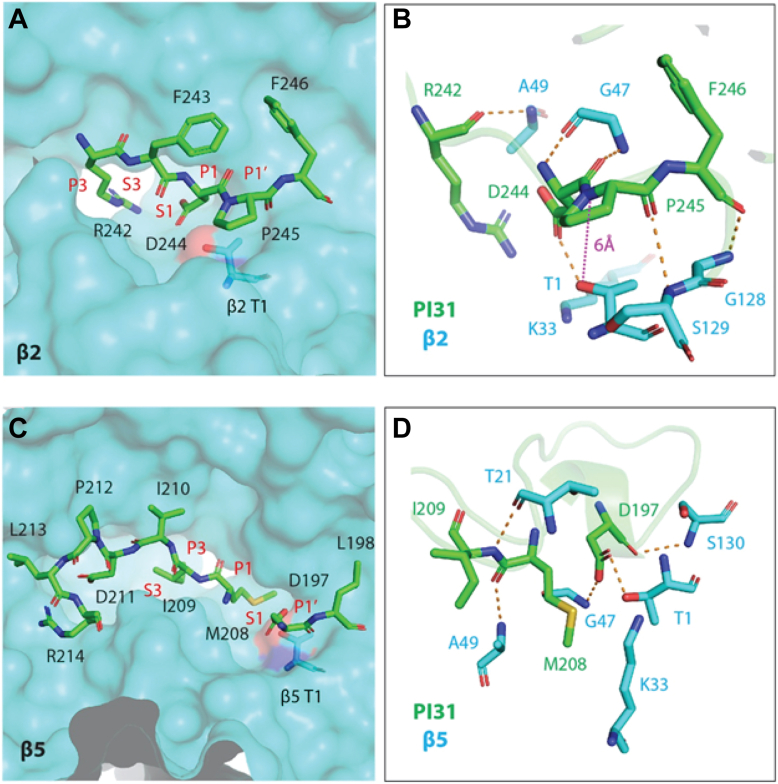
Figure 3.
PI31 mimics substrate binding to the proteasome active sites.A, binding of hPI31 to the β2 active site. The side chains of Asp244 (P1) and Arg242 (P3) are inserted into the S1 and S3 binding pockets, respectively. The Pro245 is at the P1′ position. B, PI31 in the β2 active site was stabilized by the surrounding amino acids of the β2 site. The distance between Thr1 and the cleavage site is 6 Å and may contribute to the proteolytic resistance to the β2 site. C, binding of hPI31 to the β5 active site. The side chains of M208 (P1) and I209 (P3) are inserted into the S1 and S3 binding pockets, respectively. Asp197 is at the P1′ position. D, hydrogen bonds between PI31 and the surrounding amino acids of β5 help to stabilize the bound special conformation of PI31 in β5. The special conformation leaves no peptide bond between the P1 (Met208) and P1’ (Asp197) for the β5 activity.