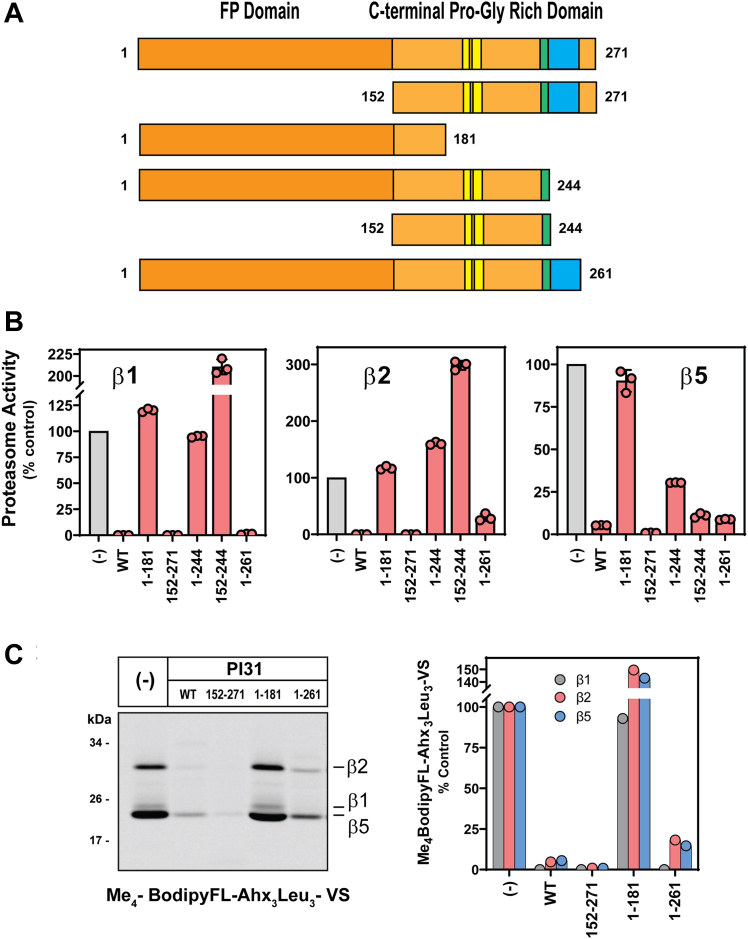
Figure 5.
Identification of PI31 interaction sites with different 20S proteasome catalytic subunit types.A, recombinant wildtype and indicated truncation mutants of hPI31 were expressed and purified as described in Experimental Procedures. Mutants are depicted schematically and show proposed interaction sites for β5, β2, and β1 catalytic sites (see Fig. 1A). The proposed interaction site for the β1 subunit is indicated in blue. B, 20S proteasome (50 nM) activity for each catalytic subunit type was measured in the absence (−) or presence of wildtype and indicated hPI31 mutants at 10- to 20-fold molar excess. Proteasome activity for each subunit type in the absence of PI31 (−) was set to 100, and other activities were expressed as a percentage of that value. Bars represent mean values (±SD) of indicated triplicate assays. Similar results were obtained in three independent experiments. Differences in proteasome activity among PI31 treatments were analyzed prior to normalization by ANOVA and Tukey’s HSD. For β1 activity, pairwise differences between all treatments were observed at p ≤ 0.0001; for β2 activity, pairwise differences between all treatments were observed at p < 0.005 except for WT versus 152 to 271 (p = 0.2353) and WT versus 1 to 262 (p = 0.0696). For β5 activity, pairwise differences between all treatments were observed at p < 0.001 except for (−) PI31 versus 1 to 181 (p = 0.0456) and WT versus 152 to 244 (p = 0.7878). C, activated 20S proteasome (115 nM) was preincubated in the absence (−) or presence of indicated hPI31 variants (10 μM) and then labeled with Me4BodipyFL-Ahx3Leu3-VS probe (500 nM) for 15 min to avoid signal saturation. Assays were quenched with SDS-PAGE sample buffer, and samples were subjected to SDS-PAGE and imaging as described in Experimental Procedures.