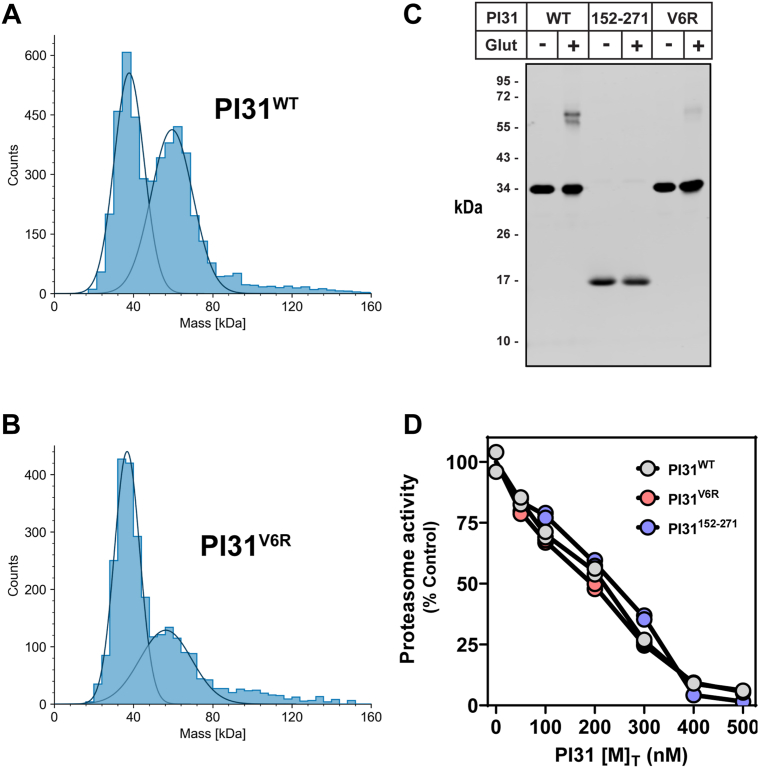
Figure 7.
PI31 monomers are sufficient to inhibit 20S proteasome activity. Mass photometry analysis of PI31 dimerization using purified, recombinant (A) hPI31WT and (B) PI31V6R dimerization mutant proteins. Proteins were diluted to 40 nM total monomer (1.2 ng μl−1) in PBS during recording and analyzed as described in Experimental Procedures. C, analysis of PI31 (WT, V6R, 152–271) dimerization by chemical cross-linking. Protein, 23 μM, was cross-linked with 0.05% glutaraldehyde or buffer controls as described in Experimental Procedures. One microgram of denatured proteins was separated by SDS-PAGE and blotted against PI31 antibodies. D, inhibition of proteasome activity by hPI31WT, hPI31V6R, and hPI3152–271. 20S proteasome (50 nM) was activated by 0.03% SDS prior to incubation with indicated amounts of total PI31 subunits, [M]T (where [M]T = [monomer] = 2 x [dimer]), up to 10-fold molar excess. Proteasome activities were measured in duplicate as described in Experimental Procedures and expressed as percentages relative to controls lacking PI31.