Abstract
Background
Cannabis is increasingly used both medically and recreationally. With widespread use, there is growing concern about how to identify cannabis-impaired drivers.
Methods
A placebo-controlled randomized double-blinded protocol was conducted to study the effects of cannabis on driving performance. One hundred ninety-one participants were randomized to smoke ad libitum a cannabis cigarette containing placebo or delta-9-tetrahydrocannabinol (THC) (5.9% or 13.4%). Blood, oral fluid (OF), and breath samples were collected along with longitudinal driving performance on a simulator (standard deviation of lateral position [SDLP] and car following [coherence]) over a 5-hour period. Law enforcement officers performed field sobriety tests (FSTs) to determine if participants were impaired.
Results
There was no relationship between THC concentrations measured in blood, OF, or breath and SDLP or coherence at any of the timepoints studied (P > 0.05). FSTs were significant (P < 0.05) for classifying participants into the THC group vs the placebo group up to 188 minutes after smoking. Seventy-one minutes after smoking, FSTs classified 81% of the participants who received active drug as being impaired. However, 49% of participants who smoked placebo (controls) were also deemed impaired at this same timepoint. Combining a 2 ng/mL THC cutoff in OF with positive findings on FSTs reduced the number of controls classified as impaired to zero, 86 minutes after smoking the placebo.
Conclusions
Requiring a positive toxicology result in addition to the FST observations substantially improved the classification accuracy regarding possible driving under the influence of THC by decreasing the percentage of controls classified as impaired.
Introduction
The relationship between cannabis use and driving impairment is complex because of the unique pharmacokinetic and pharmacodynamic properties of cannabis's major intoxicant: delta-9-tetrahydrocannabinol (THC) (1). “Impairment” is difficult to define because there is no universally agreed-upon task that can be used to define driving impairment. With ethanol there is a clear relationship between the amount of alcohol consumed, blood alcohol concentrations, and the effects on driving performance (2). With cannabis, these relationships are more complex (3). The relationship between blood THC concentrations and crash risk is not established, but there is a clear understanding that THC impairs driving performance in many, but not necessarily all, individuals (1, 4). The question that remains is how to best identify drivers who are impaired by cannabis.
There are multiple components that influence the relationship between cannabis and impairment. These include factors related to cannabis itself (e.g., % THC content), cannabis use characteristics (e.g., route of administration, frequency, and amount of exposure), characteristics of the individual using cannabis (e.g., experience, prior use), and when impairment is assessed relative to dosing. The psychoactive effects of cannabis inhalation begin within minutes of smoking or vaporization and peak within 3 hours (3), while oral administration causes effects that begin in approximately 1 hour and last up to 8 hours (5). This observation is consistent with findings showing driving-related skills recover between 3 and 5 hours after smoking cannabis (4, 6). Unlike alcohol, which is cleared within 24 hours of drinking, THC accumulates with repeated dosing, resulting in some frequent users having baseline blood concentrations >5 ng/mL. Although there may be no measurable impairment, background THC concentrations can exceed the per se driving impairment limits currently employed in some states, which are generally set at 2 or 5 ng/mL of THC in blood (7, 8). The term per se in context of driving under the influence means that when concentrations exceed the specified limit, a person is considered to be under the influence based solely on the toxicology test. After smoking, blood THC concentrations drop about 90% in the first hour (9, 10). Since it can take several hours to collect a blood specimen following a traffic stop (11), it is difficult to estimate circulating blood THC concentrations at the time of driving. In one field study of 602 cases in which drug recognition experts (DREs) determined the driver was impaired due to cannabis only (toxicology confirmed), the median THC concentration was 5.05 ng/mL—meaning that around 50% of the group were below per se limits used by some states (12).
Oral fluid (OF) is an alternative specimen that can be rapidly collected at the roadside to detect recent use of cannabis. OF has several advantages compared with analysis of blood; sample collection is noninvasive, it can be tested on screening devices at the point of contact, and, like blood, preliminary results can be confirmed by robust analytical techniques such as LC-MS/MS (13, 14). Analyzing breath samples for THC content has also been proposed for identifying recent cannabis use (15).
In the first study to evaluate police officers’ performance for detecting drug-related impairment (16), adult male participants were randomized to receive cannabis, diazepam, secobarbital, or d-amphetamine. In this study the officers were trained DREs and were blinded to the participant's study drug. Only one participant was misidentified as under the influence of cannabis, while the individual was actually administered diazepam. The ability of the officers to correctly identify cannabis exposure (sensitivity) was low but dose related, as the officers correctly identified more participants on the high THC dose as compared with the low THC dose.
Heishman et al. (17) performed a placebo-controlled double-blind study examining the ability of DREs to correctly classify participants who had been exposed to placebo, ethanol, cocaine, or THC. These authors showed that when the DRE determined impairment due to drugs other than ethanol (e.g., cocaine or THC), DRE conclusions matched toxicology results in 44% of cases. However, when considering cannabis, DREs determined that 6/16 of the participants exposed to placebo were impaired (17).
In a follow-up study, Heishman measured maximum THC plasma concentrations of 28 and 61 ng/mL 2 minutes after smoking cannabis in a low- and high-dose group, respectively (18). In this trial, more placebo-exposed participants were considered impaired than the low-dose cannabis group. These authors concluded that DRE determinations of impairment were consistent with toxicology findings in only 32% of the cases (18). The authors point out several reasons for this discrepancy including that, in the field, officers observe other clues including driving behavior, drug paraphernalia, and cannabis odor.
Most of the published research examining the reliability of DRE observations has been conducted under controlled experimental conditions and only included part of the full DRE exam. This is unavoidable in studies where participants are examined more than once because a full DRE exam typically lasts about an hour (16). This has important implications when correlating laboratory studies with field studies, because several important steps of the DRE examination, such as interviewing the arresting officer, searching the participant, examining for physical signs of drug administration, and performing a breath alcohol test, are not possible in a laboratory study conducted over multiple timepoints after smoking cannabis.
In this manuscript we report results from the largest randomized double-blinded-placebo-controlled trial to date that examines the relationship between THC concentrations in various biofluids and performance in a driving simulator. We also report on police officers’ assessment of cannabis impairment based solely on field sobriety tests (FSTs) as well as when combined with various biofluid THC concentrations for the classification of persons exposed to active drug or placebo. We report the effect of different cut-points for blood and oral fluid (OF) at different timepoints for classifying participants deemed impaired on FSTs.
Materials and Methods
Study Participants
The data presented summarize toxicology findings and FST results from a University of California San Diego Center for Medicinal Cannabis Research study (4). Briefly, 199 participants were randomized and classified as “frequent” or “occasional” users. Participants using cannabis ≥ 4 times/week were termed “frequent” users, while those with intake at least 4 times per month but < 4 times/week were termed “occasional” users. Of these 199 participants, 7 were excluded due to having > 5 ng/mL THC in OF on the day of the experimental visit, and one participant withdrew, resulting in 191 participants. Participants were randomized to smoke 700 mg of placebo (0.02% THC), 5.9% THC, or 13.4% THC cannabis in a double-blind manner. The characteristics of the dosing material were described previously (8). Participants were required to take at least 4 puffs and could smoke as much as they desired during a 10-minute smoking period. Participants were instructed to “smoke as you would at home to achieve desired highness.” The participants that smoked active drug (5.9% and 13.4% THC) were combined into a single group for all of the analyses in this report because there were no significant differences between how these 2 groups performed on the driving simulator (4) and no correlation was observed between the potential amount of THC smoked (mg based on weight returned following smoking) compared to perceived highness (10). The term “active drug” refers to participants smoking either a 5.9% or 13.4% THC cigarette.
Law Enforcement Officers
Officers (N = 11) were members of the California Highway Patrol or other California law enforcement agencies, were certified DRE instructors, and had completed DRE training according to the International Association of Chiefs of Police.
FSTS
The officers performed FSTs consisting of a walk and turn, modified Romberg, lack of convergence, one-leg stand, and finger to nose tests. These tests were described previously (19). Officers did not perform a full DRE exam due to time constraints. Based solely on participants’ performance on the FSTs, officers classified them as being impaired or not impaired.
THC Measurement
THC and related cannabinoids were quantified in blood, OF, and breath on a Waters TQ-S micro LC-MS/MS system. Analytical method details were published previously (14, 20). Precision and accuracy of THC measurements were within +/−15% with a lower limit of quantification of 0.5 ng/mL in blood, 0.4 ng/mL in OF, and 80 pg/breath pad. Blood was collected using sodium fluoride as the anticoagulant and analyzed within the time frames described by Desrosiers (21). OF was collected using the Quantisal device (Immunalysis), and analyzed within the time frames described by Scheidweiler (22). OF was collected until the blue indicator showed that 1 mL specimen was obtained or for a maximum of 10 minutes. OF results are expressed as ng/mL THC in OF. Breath samples were collected in the SensAbues drug collection device.
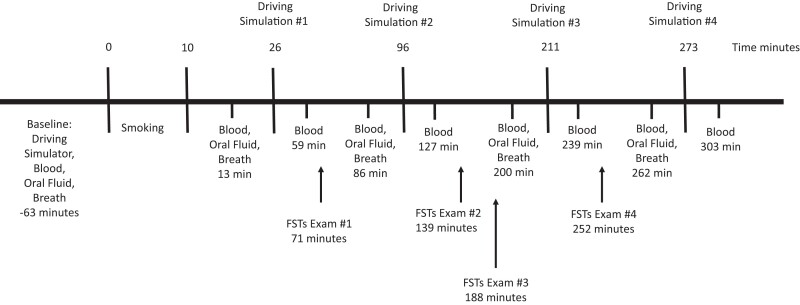
The timelines for biological specimen collections, driving simulations, and FSTs are shown in Fig. 1. The mean (SD) and median (interquartile range) times for sample collection, driving simulation, and FST exams for all subjects are shown in Supplemental Table 1. The mean (SD) and median (interquartile range) times for sample collection, driving simulation, and FST exams for subjects who smoked active drug are shown in Supplemental Table 2.
Fig. 1.
Study timeline. Times are medians since the start of the smoking period in minutes.
Driving Simulator
The driving simulator was a STISIM M300WS-Console Driving Simulator System, as described by Marcotte (4). Relationships between 2 measures of driving performance, SDLP, and car following (coherence) and THC concentrations in blood, OF, and breath are reported. SDLP, a measure of weaving in a driving lane, and coherence, a measure of ability to maintain a consistent distance with a leading vehicle, are 2 commonly used indicators of impairment (23–25). An increase in SDLP indicates worse driving performance while a decrease in coherence indicates worse driving performance.
Determination of Impairment
There is no reference method for identifying impairment following use of cannabis. Real-world driving impairment is the ultimate outcome of interest, but that is difficult to operationally define (crashes, high-risk behaviors, or slowed response to obstacles). Driving simulations, cognitive testing, and FSTs are all surrogates of impairment. Impairment was defined as the officer's interpretation of the participant's performance across all of the FSTs. Any participant with sufficient deficits on the FSTs that a trained police officer deemed them unsafe to drive was defined as being impaired. This definition was selected because, in a traffic stop, an officer's observation of driving performance is the precipitating event, followed by FSTs and possibly a DRE exam. Unlike a traffic stop, in this controlled study officers did not observe participants’ driving and made a determination of impairment based only on observations during the FST examination. Results from the FSTs were combined with different cutoff blood or OF THC concentrations to classify participants as impaired, as was done previously (26).
Statistics
Correlation between driving performance (SDLP and coherence) and THC concentrations in blood, OF, and breath were determined using Spearman rho. P values were adjusted (padj) for multiple testing using the false discovery rate method. P values are from χ2 tests for comparing proportions of impaired (or, equivalently, proportions of nonimpaired) between the THC and placebo groups. P values < 0.05 were considered significant.
Results
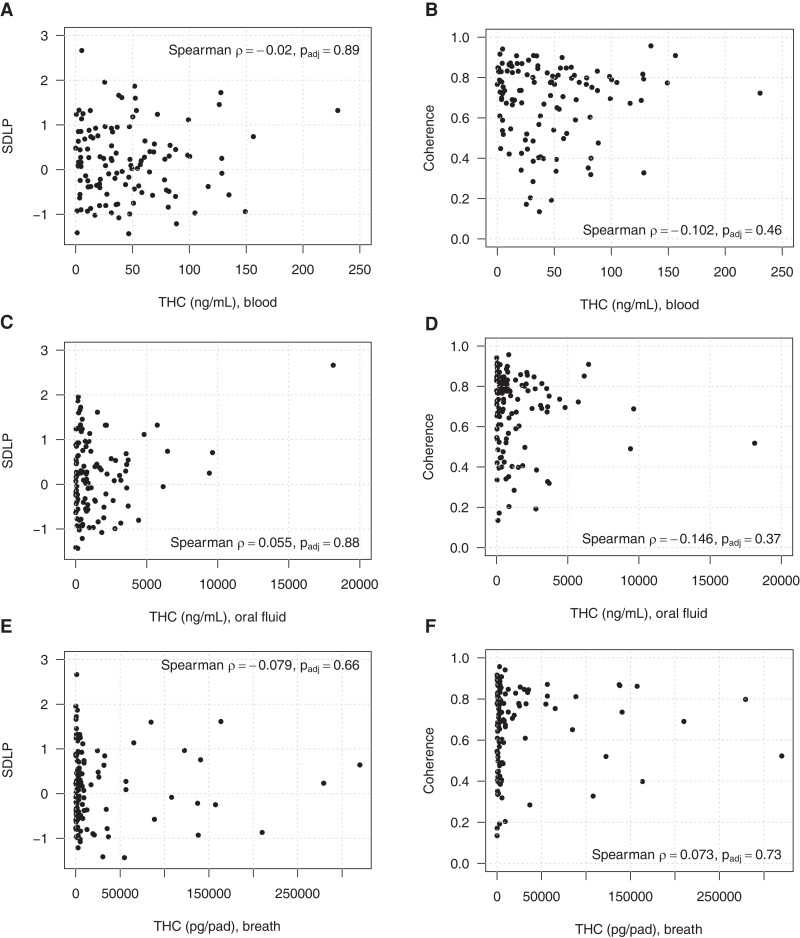
The relationship between THC concentrations in blood, OF, and breath obtained immediately after smoking active drug (i.e., peak measured concentration, median of 13 minutes post-smoking) vs driving performance at the first post-smoking driving simulation (median 26 minutes post-smoking) are shown in Fig. 2. No correlation was observed between blood THC concentration and SDLP (Fig. 2, A, rho = −0.02, padj = 0.89) or coherence (Fig. 2, B; r = −0.102, P = 0.46). These same parameters (SDLP and coherence) compared to THC concentrations measured immediately after the driving simulator (60 minutes post-smoking) also showed no relationship. The same results were observed for all other time points, as well as for analyses comparing THC concentrations and changes in simulator performance between the pre-smoking and post-smoking simulations (data not shown). We also analyzed the OF and breath data in a similar manner (Fig. 2, C–F) and demonstrated no relationship between biofluid concentrations and driving performance. The data in Table 1 shows the Spearman correlation (P values) between biofluids and driving performance and are similar to the data shown in Fig. 2 but include all of the time points in the study, vs Fig. 2, which only shows data for the first driving simulation. The data in Table 1 are important because drivers could potentially be stopped at various times after smoking cannabis. Table 1 shows that biofluid results from immediately preceding the simulator had no correlation (all P values > 0.05) with driving performance at any time during the study for the active drug group. Table 2 is included because, in the field, driving performance is observed prior to collection of biofluid specimens, which are often obtained several hours after the traffic stop. Here we show that the initial driving performance (26 minutes) did not correlate with blood, OF, or breath at any collection period (13, 86, 200, or 262 minutes after smoking) for the active drug group. Both tables show no relationship between biofluid concentrations and driving performance at any time point.
Fig. 2.
Relationship of peak THC concentration in blood, oral fluid, and breath obtained a median of 13 minutes post-smoking active drug vs performance in driving simulation at 26 minutes. None of the relationships depicted were significant. (A), Correlation between blood THC concentrations and SDLP, an indication of swerving; (B), Correlation between blood THC concentrations and car following (coherence); (C), Correlation between oral fluid THC concentrations and SDLP; (D), Correlation between oral fluid THC concentrations and coherence; (E), Correlation between breath THC and SDLP; (F), Correlation between breath THC and coherence.
Table 1.
Spearman correlations (P value) between THC concentrations in various biofluids and driving simulation outcomes among participants in the active drug group. For these analyses, THC biospecimen concentrations collected just prior to the driving simulation are evaluated against SDLP and coherence (car following). P values were adjusted for multiple testing using the false discovery rate method.
| Simulation timepoint |
Blood THC correlation (P value) |
Oral fluid THC correlation (P value) |
Breath THC correlation (P value) |
|---|---|---|---|
| SDLP | |||
| Baseline | −0.013 (0.887) | −0.055 (0.882) | −0.057 (0.680) |
| Simulation 1 | −0.020 (0.887) | 0.055 (0.882) | −0.079 (0.663) |
| Simulation 2 | −0.062 (0.874) | −0.046 (0.882) | 0.036 (0.700) |
| Simulation 3 | −0.221 (0.134) | −0.014 (0.882) | 0.080 (0.663) |
| Simulation 4 | −0.160 (0.286) | 0.023 (0.882) | −0.089 (0.663) |
| Coherence | |||
| Baseline | −0.079 (0.495) | 0.044 (0.719) | −0.048 (0.764) |
| Simulation 1 | −0.102 (0.460) | −0.146 (0.368) | 0.073 (0.728) |
| Simulation 2 | −0.122 (0.460) | 0.033 (0.719) | 0.078 (0.728) |
| Simulation 3 | −0.112 (0.460) | −0.136 (0.368) | 0.018 (0.845) |
| Simulation 4 | 0.039 (0.699) | 0.078 (0.673) | −0.153 (0.510) |
Table 2.
Spearman correlations (P value) between THC concentrations and driving simulation outcomes among participants in the active drug group. For this analysis, SDLP and coherence results from the first post-smoking simulated driving session (26 minutes) are evaluated against all biofluid samples. P values were adjusted for multiple testing using the false discovery rate method.
| Timepoint (min) (fluid collection) |
Blood THC correlation (P value) | Oral fluid THC correlation (P value) | Breath THC correlation (P value) |
|---|---|---|---|
| SDLP | |||
| 13 | −0.020 (0.835) | 0.055 (0.576) | −0.079 (0.531) |
| 86 | −0.074 (0.596) | 0.052 (0.576) | 0.051 (0.585) |
| 200 | −0.092 (0.596) | 0.088 (0.576) | −0.096 (0.531) |
| 262 | −0.107 (0.596) | 0.057 (0.576) | −0.200 (0.121) |
| Coherence | |||
| 13 | −0.102 (0.528) | −0.146 (0.114) | 0.073 (0.683) |
| 86 | −0.062 (0.528) | −0.188 (0.074) | −0.038 (0.683) |
| 200 | 0.073 (0. 528) | −0.178 (0.074) | −0.096 (0.683) |
| 262 | 0.102 (0. 528) | −0.219 (0.068) | −0.041 (0.683) |
In addition to THC, we also measured cannabinol, cannabidiol, 11-hydroxy-THC, 11-nor-9-carboxy-delta-9-THC, 11-nor-9-carboxy-delta-9-THC-9-carboxylic acid glucuronide, cannabigerol, delta-9-THC glucuronide, delta-9-tetrahydrocannabiniolic acid, and tetrahydrocannabivarin (14, 20). None of the 10 cannabinoids in our analysis correlated with the simulator driving performance (SDLP or coherence) at any time point.
Table 3 shows results of FST determinations of impairment for participants who smoked placebo or active drug. Only participants who had FSTs examined at all time points (63 placebo and 121 active drug) were included. At FST exam #1, performed a median of 71 minutes from the start of smoking, 98/121 participants (81.0%) who received active drug were classified as impaired. At the same time point, 31/63 participants who smoked placebo were classified as impaired (49.2%). There was a significant difference between participants who smoked active drug that were classified as impaired by the FSTs vs placebo for the first 3 FST examinations. By 252 minutes post start of smoking, there was no significant difference between those who received active drug vs placebo for FST classification of impairment. As the study day progressed, lower percentages of both active drug and placebo participants were considered impaired. Detailed analyses and discussion regarding the FSTs, overall driving performance, and characterization of the placebo group are addressed in Marcotte et al. (27).
Table 3.
FST determination of impairment for active drug (THC) or placebo, shown as a percentage. P values were adjusted for multiple testing using the false discovery rate method.
| FSTs exam # Outcome |
THC | Placebo | P |
|---|---|---|---|
| Exam 1 | <0.001 | ||
| Impaired (%) | 81.0 | 49.2 | |
| Not impaired (%) | 19.0 | 50.8 | |
| Exam 2 | <0.001 | ||
| Impaired (%) | 62.5 | 28.6 | |
| Not impaired (%) | 37.5 | 71.4 | |
| Exam 3 | 0.032 | ||
| Impaired (%) | 36.4 | 19.0 | |
| Not impaired (%) | 63.6 | 81.0 | |
| Exam 4 | 0.160 | ||
| Impaired (%) | 22.5 | 12.7 | |
| Not impaired (%) | 77.5 | 87.3 |
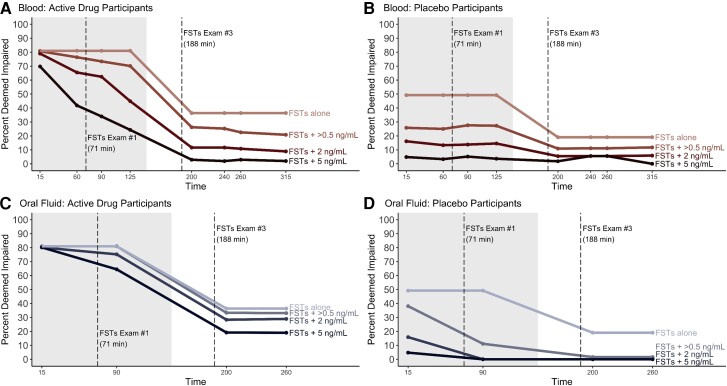
Figure 3 shows how adding a requirement for a positive toxicology test in addition to the FST results changes the classification of impairment. For this figure we evaluate FST results alone (top line in graph) along with 3 different THC cutoff concentrations (>0.5 ng/mL, 2 ng/mL, and 5 ng/mL). For this analysis we applied the FST assessment from 2 different time points. We chose FST exam #1 (shaded area, 71 minutes post-smoking) and FST exam #3 (188 minutes post-smoking) because the first represents the FST examination at the highest THC concentration and the latter is about 3 hours after smoking when effects should be starting to wear off (6). Figure 3, A shows that at 90 minutes after smoking, when a 2 ng/mL blood THC cutoff is required in addition to the FST results, 62.4% of subjects who received active drug met both criteria, compared to 81.0% when just the FSTs were employed. Figure 3, B shows that, for the placebo group, requiring a 2 ng/mL blood cutoff decreased the percent of the placebo cohort classified as impaired to 13.8% at 90 minutes post-smoking compared to 49.2% when just the FSTs were used. The placebo group exceeding the toxicology thresholds reflect their baseline THC concentrations, as might be encountered in the field for drivers who use cannabis, even though it was not recent (i.e., greater than 48 hours before the collection) (7). Figure 3, C shows that at 90 minutes after smoking, when a 2 ng/mL OF THC cutoff is required in addition to the FST results, 75.2% of active drug subjects met both criteria vs 81.0% when just the FSTs were used. Figure 3, D shows that requiring a 2 ng/mL OF cutoff decreased the number of the placebo cohort who were classified as impaired to 0% at 90 minutes post-smoking vs 49.2% when just the FSTs were used. Supplemental Table 3 shows the effect of combining different toxicology concentration cutoffs (OF and blood) and FST results for classifying the active drug and placebo cohorts for all time points. We did not include the effect of adding breath samples to FST examinations because our previously published results (10, 11) showed that, using the breath collection device we employed, THC rapidly dissipates and by the second post-smoking time point was undetectable in most participants.
Fig. 3.
Effect of adding toxicology testing for THC at various cutoffs (ng/mL) to determinations of impairment based solely on FSTs at various timepoints after smoking. The shaded area refers to where the specific toxicology cutoffs are applied to FSTs Exam #1 (71 minutes post-smoking); the unshaded area represents FSTs Exam #3 results (188 minutes post-smoking) combined with specific toxicology cutoff concentrations. Dashed lines indicate time of the FSTs. (A), Percent of active drug group classified as impaired and exceeding specific blood cutoff concentrations; (B), Percent of placebo group classified as impaired and exceeding specific blood cutoff concentrations; (C), Percent of active drug group classified as impaired and exceeding specific oral fluid cutoff concentrations; (D), Percent of placebo group classified as impaired and exceeding specific oral fluid cutoff concentrations.
Discussion
Biofluid THC concentrations, as well as other cannabinoid concentrations, did not correlate with SDLP or coherence at any time point. The lack of correlation between THC and driving performance on a simulator was reported previously in studies up to about 3.5 hours after inhaling THC (4, 26). Previously we reported that the composite driving score, a combined measure of impairment, did not correlate to blood THC concentrations (4). Since SDLP and coherence are 2 of the most commonly reported simulator results sensitive to cannabis (25, 28), details of these findings in relation to blood, OF, and breath THC concentrations are reported. The complete lack of a relationship between the concentration of the centrally active component of cannabis in blood, OF, and breath is strong evidence against the use of per se laws for cannabis. Our results are consistent with a recent meta-analysis that concentrations of THC are “relatively poor indicators of cannabis-induced impairment” (29). Unlike ethanol (30, 31), there is no established relationship between blood THC concentrations and simulator driving performance measures (4, 26). In the largest randomized double-blinded placebo-controlled trial to date, our data confirm that THC concentrations (and/or metabolites/related cannabinoids) in blood, OF, or breath cannot be used as a sole indicator of impairment.
Previously we reported that participants smoking active cannabis produced a significant (P < 0.05) decrement in driving performance that lasted for about 3.5 hours (4). Thus, when evaluating the ability of FSTs to detect impairment, it is expected that they classify more participants exposed to active drug as being impaired early following cannabis inhalation as opposed to later time points. Table 3 shows that FSTs classified 81.0% of the active drug participants as impaired at the first FSTs exam while only 22.5% were classified as being impaired at the last time point (251 minutes post-smoking). As evidenced by driving performance in the simulator (4), and results from the FSTs exam, not all participants who smoked active drug were impaired at the time of the FSTs. The relationships between simulator driving performance and individual FST results are reported in a separate publication (27).
Of note, in isolation (e.g., without driving observations or a full DRE exam or toxicology testing), the FSTs classified 49.2% of the placebo group as impaired. This is consistent with previous reports from smaller studies examining the ability of FSTs to identify impairment following cannabis use (17, 18). Practice effects were limited in this study compared to previous studies that exposed participants to FSTs prior to administering study drug. In our study, participants were first exposed to FSTs after the smoking session. This likely increased the sensitivity of the FSTs to active drug but also could have contributed to the high number of placebo participants who were categorized as impaired.
Adding a requirement for a positive toxicology test to the FST exam results slightly decreased the percentage of participants who smoked active drug that were classified as being impaired but dramatically decreased the percentage of placebo group subjects that were classified as impaired. When the same cutoff concentration was used, OF showed less of an impact than blood for reclassifying the active drug cohort while reclassifying a higher percentage of the placebo group as not impaired. While adding toxicology results may be helpful in increasing the level of suspicion that cannabis was involved in driving impairment, they do not demonstrate causality. Therefore, the results of this study do not translate into supporting per se approaches.
Limitations
When interpreting these results, several factors need to be considered. First, when relating THC biofluid concentrations to driving performance, our participants’ driving skills were evaluated using a driving simulator in a controlled environment that does not reflect all of the variables that confound actual driving. Second, our participants were instructed not to use cannabis for at least 2 days prior to the study and were excluded from the study if their OF contained ≥ 5 ng/mL THC at baseline. This likely complicates the application of our results to real-world data where participants frequently smoke more than one cannabis cigarette, with chronic users smoking multiple cannabis cigarettes on a daily basis. Third, the same officer examined each participant at multiple time points, which could influence the interpretation of impairment at later time points. In addition, officers also knew the participants would be under the influence of cannabis alone or placebo. Finally, a full DRE exam was not performed, and impairment was determined based solely on the FSTs results.
Conclusions
In the largest trial to date involving experienced users smoking cannabis, there was no correlation between THC (and related metabolites/cannabinoids) in blood, OF, or breath and driving performance. Our data support the current practice in many areas of the United States that requires officer observations of impairment along with toxicology testing before prosecuting drivers for being under the influence. We provide some evidence for the use of OF as opposed to blood as being more useful in reducing the likelihood of false accusations of driving under the influence of cannabis. The selection of an optimal cutoff is an important determinant of road safety that deserves further study. A better understanding of how a full DRE exam compares with FSTs is also warranted.
Supplemental Material
Supplemental material is available at Clinical Chemistry online.
Supplementary Material
Acknowledgments
The authors would like to thank Barth Wilsey, MD (retired), for his integral role in the development and early success of the project. In addition, we thank Sandra Sanford; Robert Bryan; Jennifer Marquie-Beck, MPH; Clint Cushman, BA; Donald Franklin Jr., BA; Haley Ceremony, BA; Julia Drizin, BA; and Alejandra Vidrio, BA for assistance in study coordination and data collection. We wish to acknowledge the University of California San Diego Center for Medicinal Cannabis Research, which contributed facilities, core staff, and other infrastructure support to this project.
Contributor Information
Robert L Fitzgerald, Center for Advanced Laboratory Medicine, San Diego, CA, United States.
Anya Umlauf, Department of Psychiatry, University of California San Diego, Center for Medicinal Cannabis Research, San Diego, CA, United States.
Jacqueline A Hubbard, Qualitox Labs, McKees Rocks, PA, United States.
Melissa A Hoffman, Vividion Therapeutics, San Diego, CA, United States.
Philip M Sobolesky, Santa Clara Valley Medical Center, San Jose, CA, United States.
Shannon E Ellis, Department of Cognitive Sciences, University of California San Diego, La Jolla, CA, United States.
David J Grelotti, Department of Psychiatry, University of California San Diego, Center for Medicinal Cannabis Research, San Diego, CA, United States.
Raymond T Suhandynata, Center for Advanced Laboratory Medicine, San Diego, CA, United States.
Marilyn A Huestis, Institute for Emerging Health Professions, Thomas Jefferson University, Philadelphia, PA, United States.
Igor Grant, Department of Psychiatry, University of California San Diego, Center for Medicinal Cannabis Research, San Diego, CA, United States.
Thomas D Marcotte, Department of Psychiatry, University of California San Diego, Center for Medicinal Cannabis Research, San Diego, CA, United States.
Nonstandard Abbreviations
THC, delta-9-tetrahydrocannabinol; OF, oral fluid; SDLP, standard deviation of lateral position; FST, field sobriety test; DRE, drug recognition expert; coherence, car following.
Author Contributions
The corresponding author takes full responsibility that all authors on this publication have met the following required criteria of eligibility for authorship: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; (c) final approval of the published article; and (d) agreement to be accountable for all aspects of the article thus ensuring that questions related to the accuracy or integrity of any part of the article are appropriately investigated and resolved. Nobody who qualifies for authorship has been omitted from the list.
Robert Fitzgerald (Conceptualization-Equal, Data curation-Equal, Formal analysis-Equal, Investigation-Equal, Methodology-Equal, Resources-Supporting, Supervision-Equal, Validation-Equal, Writing—original draft-Lead, Writing—review & editing-Lead), Anya Umlauf (Data curation-Equal, Formal analysis-Equal), Jacqueline Hubbard (Data curation-Supporting, Formal analysis-Supporting, Methodology-Supporting, Writing—review & editing-Supporting), Melissa Hoffman (Data curation-Supporting, Formal analysis-Supporting, Methodology-Supporting, Validation-Supporting), Philip Sobolesky (Data curation-Supporting, Formal analysis-Supporting, Methodology-Supporting), Shannon Ellis (Data curation-Equal, Formal analysis-Supporting, Writing—review & editing-Supporting), David Grelotti (Conceptualization-Equal, Supervision-Equal, Writing—review & editing-Supporting), Raymond Suhandynata (Data curation-Equal, Methodology-Supporting, Writing—review & editing-Supporting), Marilyn Huestis (Conceptualization-Supporting, Methodology-Supporting, Writing—review & editing-Supporting), Igor Grant (Conceptualization-Supporting, Funding acquisition-Equal, Methodology-Equal, Writing—review & editing-Supporting), and Thomas Marcotte (Conceptualization-Lead, Data curation-Equal, Formal analysis-Equal, Funding acquisition-Equal, Investigation-Equal, Methodology-Equal, Project administration-Equal, Supervision-Lead, Writing—original draft-Equal, Writing—review & editing-Equal)
Authors’ Disclosures or Potential Conflicts of Interest
Upon manuscript submission, all authors completed the author disclosure form. Disclosures and/or potential conflicts of interest:
Employment or Leadership
None declared.
Consultant or Advisory Role
M.A. Huestis, Canadian Nuclear Safety Commission, Hound Labs, Federal Aviation Agency, Safe Approaches to Marijuana.
Stock Ownership
None declared.
Honoraria
None declared.
Research Funding
This work was supported by the State of California award to the Center for Medicinal Cannabis Research via Assembly Bill 266 (Bonta/Cooley/Jones-Sawyer/Lackey: Agreement #907). R.L. Fitzgerald reported grants from the State of California and from the National Institutes of Health during the conduct of the study. R.L. Fitzgerald reports funding from the American Association for Clinical Chemistry to present research results. A. Umlauf reported grants from the State of California during the conduct of this study. M.A. Hoffman disclosed funding from the State of California. D.J. Grelotti reported funding from the State of California during the conduct of the study. M.A. Huestis reported funding from the Canadian Nuclear Safety Commission, the Federal Aviation Agency, and Hound Laboratories. I. Grant reported grants from the State of California and from the National Institutes of Health during the conduct of the study. T.D. Marcotte reported grants from the State of California and from the National Institutes of Health during the conduct of the study. T.D. Marcotte also reports funding from the American Association for Clinical Chemistry to present research results.
Expert Testimony
None declared.
Patents
None declared.
Other Remuneration
R.L. Fitzgerald and T.D. Marcotte, support for attending meetings and/or travel from AACC; P. Sobolesky, support for attending meetings and/or travel from The Association for Mass Spectrometry & Advances in the Clinical Lab; R. Suhandynata, MSACL Educational Grant recipient.
Role of Sponsor
The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, preparation of manuscript, or final approval of manuscript.
References
- 1. Hartman RL, Huestis MA. Cannabis effects on driving skills. Clin Chem 2013;59:478–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Martin TL, Solbeck PA, Mayers DJ, Langille RM, Buczek Y, Pelletier MR. A review of alcohol-impaired driving: the role of blood alcohol concentration and complexity of the driving task. J Forensic Sci 2013;58:1238–50. [DOI] [PubMed] [Google Scholar]
- 3. Ramaekers JG, Moeller MR, van Ruitenbeek P, Theunissen EL, Schneider E, Kauert G. Cognition and motor control as a function of delta9-THC concentration in serum and oral fluid: limits of impairment. Drug Alcohol Depend 2006;85:114–22. [DOI] [PubMed] [Google Scholar]
- 4. Marcotte TD, Umlauf A, Grelotti DJ, Sones EG, Sobolesky PM, Smith BE, et al. Driving performance and cannabis users’ perception of safety: a randomized clinical trial. JAMA Psych 2022;79:201–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grotenhermen F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet 2003;42:327–60. [DOI] [PubMed] [Google Scholar]
- 6. McCartney D, Arkell TR, Irwin C, McGregor IS. Determining the magnitude and duration of acute Δ9-tetrahydrocannabinol (Δ9-THC)-induced driving and cognitive impairment: a systematic and meta-analytic review. Neurosci Biobehav Rev 2021;23:175–93. [DOI] [PubMed] [Google Scholar]
- 7. Bergamaschi MM, Karschner EL, Goodwin RS, Scheidweiler KB, Hirvonen J, Queiroz RH, et al. Impact of prolonged cannabinoid excretion in chronic daily cannabis smokers’ blood on per se drugged driving laws. Clin Chem 2013;59:519–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huestis MA, Henningfield JE, Cone EJ. Blood cannabinoids. I. Absorption of THC and formation of 11-OH-THC and THCCOOH during and after smoking marijuana. J Anal Toxicol 1992;16:276–82. [DOI] [PubMed] [Google Scholar]
- 9. Hubbard JA, Hoffman MA, Ellis SE, Sobolesky PM, Smith BE, Suhandynata RT, et al. Biomarkers of recent cannabis use in blood, oral fluid and breath. J Anal Toxicol 2021;45:820–8. [DOI] [PubMed] [Google Scholar]
- 10. Hoffman MA, Hubbard JA, Sobolesky PM, Smith BE, Suhandynata RT, Sanford A, et al. Blood and oral fluid cannabinoid profiles of frequent and occasional cannabis smokers. J Anal Toxicol 2021;45:851–62. [DOI] [PubMed] [Google Scholar]
- 11. Hartman RL, Brown TL, Milavetz G, Spurgin A, Gorelick DA, Gaffney GR, et al. Effect of blood collection time on measured Δ9-tetrahydrocannabinol concentrations: implications for driving interpretation and drug policy. Clin Chem 2016;62:367–77. [DOI] [PubMed] [Google Scholar]
- 12. Logan B, Kacinko SL, Beirness DJ. An evaluation of data from drivers arrested for driving under the influence in relation to per se limits for cannabis. Washington (DC): AAA Foundation for Traffic Safety; 2016. [Google Scholar]
- 13. Truver MT, Palmquist KB, Swortwood MJ. Oral fluid and drug impairment: pairing toxicology with drug recognition expert observations. J Anal Toxicol 2019;43:637–43. [DOI] [PubMed] [Google Scholar]
- 14. Sobolesky PM, Smith BE, Hubbard JA, Stone J, Marcotte TD, Grelotti DJ, et al. Validation of a liquid chromatography-tandem mass spectrometry method for analyzing cannabinoids in oral fluid. Clin Chim Acta 2019;491:30–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Himes SK, Scheidweiler KB, Beck O, Gorelick DA, Desrosiers NA, Huestis MA. Cannabinoids in exhaled breath following controlled administration of smoked cannabis. Clin Chem 2013;59:1780–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bigelow GE, Bickel WE, Liebson IA, Nowowieski P. Identifying types of drug intoxication: laboratory evaluation of a subject-examination procedure (DOT HS 806 753). Washington (DC): National Highway Traffic Safety Administration; 1985. [Google Scholar]
- 17. Heishman SJ, Singleton EG, Crouch DJ. Laboratory validation study of drug evaluation and classification program: ethanol, cocaine, and marijuana. J Anal Toxicol 1996;20:468–83. [DOI] [PubMed] [Google Scholar]
- 18. Heishman SJ, Singleton EG, Crouch DJ. Laboratory validation study of drug evaluation and classification program: alprazolam, d-amphetamine, codeine, and marijuana. J Anal Toxicol 1998;22:503–14. [DOI] [PubMed] [Google Scholar]
- 19. Declues K, Perez S, Figueroa A. A 2-year study of delta 9-tetrahydrocannabinol concentrations in drivers: examining driving and field sobriety test performance. J Forensic Sci 2016;61:1664–70. [DOI] [PubMed] [Google Scholar]
- 20. Hubbard JA, Smith BE, Sobolesky PM, Kim S, Hoffman MA, Stone J, et al. Validation of a liquid chromatography tandem mass spectrometry (LC-MS/MS) method to detect cannabinoids in whole blood and breath. Clin Chem Lab Med 2020;58:673–81. [DOI] [PubMed] [Google Scholar]
- 21. Desrosiers NA, Lee D, Scheidweiler KB, Concheiro-Guisan M, Gorelick DA, Huestis MA. In vitro stability of free and glucuronidated cannabinoids in urine following controlled smoked cannabis. Anal Bioanal Chem 2014;406:785–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Scheidweiler KB, Andersson M, Swortwood MJ, Sempio C, Huestis MA. Long-term stability of cannabinoids in oral fluid after controlled cannabis administration. Drug Test Anal 2017;9:143–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hartley S, Simon N, Larabi A, Vaugier I, Barbot F, Quera-Salva MA, et al. Effect of smoked cannabis on vigilance and accident risk using simulated driving in occasional and chronic users and the pharmacokinetic-pharmacodynamic relationship. Clin Chem 2019;65:684–93. [DOI] [PubMed] [Google Scholar]
- 24. Doroudgar S, Mae Chuang H, Bohnert K, Canedo J, Burrowes S, Perry PJ. Effects of chronic marijuana use on driving performance. Traffic Inj Prev 2018;19:680–6. [DOI] [PubMed] [Google Scholar]
- 25. Hartman RL, Brown TL, Milavetz G, Spurgin A, Pierce RS, Gorelick DA, et al. Cannabis effects on driving lateral control with and without alcohol. Drug Alcohol Depend 2015;154:25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Arkell TR, Spindle TR, Kevin RC, Vandrey R, McGregor IS. The failings of per se limits to detect cannabis-induced driving impairment: results from a simulated driving study. Traffic Inj Prev 2021;22:102–7. [DOI] [PubMed] [Google Scholar]
- 27. Marcotte TD, Umlauf A, Grelotti DJ, Sones EG, Mastropietro KF, Suhandynata RT, et al. Evaluation of the field sobriety tests in identifying drivers under the influence of cannabis: A randomized clinical trial. Forthcoming. [DOI] [PMC free article] [PubMed]
- 28. Arkell TR, McCartney D, McGregor IS. Medical cannabis and driving. Aust J Gen Pract 2021;50:357–62. [DOI] [PubMed] [Google Scholar]
- 29. McCartney D, Arkell TR, Irwin C, Kevin RC, McGregor IS. Are blood and oral fluid Δ9-tetrahydrocannabinol (THC) and metabolite concentrations related to impairment? A meta-regression analysis. Neurosci Biobehav Rev 2022;134:1–10. [DOI] [PubMed] [Google Scholar]
- 30. Yadav AK, Velaga NR. Effect of alcohol use on accelerating and braking behaviors of drivers. Traffic Inj Prev 2019;20:353–8. [DOI] [PubMed] [Google Scholar]
- 31. Irwin C, Iudakhina E, Desbrow B, McCartney D. Effects of acute alcohol consumption on measures of simulated driving: a systematic review and meta-analysis. Accid Anal Prev 2017;102:248–66. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.