Abstract
Objective
Obesity is a significant risk factor for metabolic syndrome, type 2 diabetes mellitus, hypertension, nonalcoholic fatty liver disease, and cardiovascular disorders. As a well-known Chinese tea product, Besunyen Slimming Tea (BST) is believed to effectively reduce body weight (BW) and lipid profile. In this study, we aimed to elucidate the mechanisms and effects of BST on treating obesity and hepatic steatosis using a rat model fed with a high-fat diet (HFD).
Methods
Sprague-Dawley rats were subjected to random separation into three categories: Animals were fed (1) a normal diet food (ND); (2) HFD, and (3) HFD BST (n = 12/category). After successfully establishing the obesity model at week 8, the HFD BST received BST (0.6 g/0.6 kg) orally, and the ND and HFD received the same amount (2 ml) of distilled water orally.
Results
HFD BST reduced waist circumference (7.84%, P 0.015), food intake (14.66%, P 0.011), final BW (12.73%, P 0.010), BW gain (964.16%, P 0.001), and body mass index (8.97%, P 0.044) compared with the HFD. BST supplementation also decreased hyperlipidemia, inflammation, and insulin resistance in rats with HFD. Furthermore, BST suppressed hepatic lipidosis by decreasing de novo lipogenesis and increasing fatty acid oxidation.
Conclusions
The results of this study offer evidence supporting the potential health benefits of BST in the management of metabolic disorders and obesity.
Keywords: Obesity, High fat diet, Tea polyphenols, Flavonoids, Liver, Lipogenesis, Fatty acid oxidation
1. Introduction
Obesity is a growing worldwide health concern often correlated with dyslipidemia, insulin resistance, oxidative stress, and inflammation. Obesity is characterized by storing extra adipose in the body because of the imbalance between energy consumption and intake, and a threatening element for cardiovascular disease, hypertension, type 2 diabetes mellitus, metabolic syndrome, and nonalcoholic fatty liver disease (NAFLD) [[1], [2], [3], [4], [5]].
Hepatolipidosis and NAFLD are characterized by excessive liver triglyceride (TG) accumulation and elevated serum aspartate transaminase and alanine transaminase (ALT). Activation of the transcription factor sterol regulatory element binding protein1c (SREBP1c) up-regulates fatty acid synthase (FAS) and acetyl-coenzyme A carboxylase (ACC), which may promote de novo lipogenesis [[6], [7], [8], [9], [10]]. In addition, peroxisome proliferator-activated receptorα (PPARα) and carnitine palmitoyltransferase1α (CPT1α) may suppress hepatic lipid accumulation and promote β-oxidation of fatty acid. AMP-activated protein kinase (AMPK) is an essential homeostatic factor in energy metabolism. AMPK phosphorylation and activation inhibit anabolic pathways, such as the synthesis of fatty acid, and promote catabolic pathways, such as fatty acid oxidation. AMPK activation up-regulates CPT1α and PPARα to decrease the accumulation of hepatic lipids, increases β-oxidation of fatty acid, and down-regulates FAS, ACC, and SREBP1c to suppress de novo lipogenesis. Thus, AMPK has been identified as a critical target in managing obesity and hepatic adiposity [3,[6], [7], [8], [9],[11], [12], [13], [14]].
Obesity control measures such as lifestyle modification (reducing energy intake and increasing physical activity), drug therapy, and bariatric surgery remain ineffective and have side effects. Therefore, the search for other safe and effective anti-obesity methods is of great importance, especially functional ingredients derived from natural dietary sources have been the focus of research [1,5,15,16]. Polyphenols and flavonoids may regulate glucose and lipid metabolism, oxidative stress, and inflammation, effectively preventing obesity and liver adiposity. In many cases, the beneficial effects of polyphenols and flavonoids involve the activation of AMPK [[2], [3], [4],[17], [18], [19], [20], [21], [22], [23]].
Tea can be categorized into several types: unfermented green tea, lightly fermented white and yellow tea, semi-fermented oolong tea, deeply fermented black tea, and post-fermented dark tea [24]. The manufacturing and fermentation processes produce teas with varying chemical compositions and biological properties [25]. For instance, kombucha is a beverage whose bioactive properties are enhanced by the symbiotic culture of bacteria and yeast fermentation process [[26], [27], [28]]. Dark tea, such as Yunnan pu-erh tea and Hunan Fu-Zhuan tea, is a post-fermented tea produced through a unique stacked fermentation process involving microorganisms like Aspergillus niger. Due to this distinctive production method, dark tea contains different bioactive components than other teas, such as theaflavins, thearubigins, and theabrownins, oxidized and polymerized derivatives of tea polyphenols [29,30]. A well-known Chinese tea product, Besunyen Slimming Tea (BST), is believed to reduce body weight (BW) and lipid profile due to tea polyphenols and flavonoids. Therefore, here, the mechanisms and effects of BST were evaluated on the medication of obesity and hepatic lipidosis in a rodent model induced by a high fat diet (HFD).
2. Materials and methods
2.1. BST information
BST was purchased from “Besunyen Holdings, Inc”. Its main composition is senna leaves, honeysuckle, Gynostemma pentaphyllum, green tea, cassia spirit, lotus leaves, hawthorn, and honey. Ingredients include tea polyphenols (1.33 g/100 g) and total flavonoids (0.42 g/100 g) (www.besunyen.com). The BST solution was prepared following the manufacturer-recommended protocols. Briefly, 2 BST teabags (2.5 g/bag) were added to boiling water for 5–8 min, twice daily to a 60 kg adult (2.5 g 2 2/60 kg 10 g/60 kg), 3 BST teabags were added to 25 ml boiling water for 5–8 min, then to a 0.6 kg rat 2 ml daily gavage (2.5 g 3 2/25/0.6 kg 0.6 g/0.6 kg). Ultra-high performance liquid chromatography (UHPLC) (Thermo Scientific™ UltiMate™ 3000 Rapid Separation Dual System) was used to analyze the BST solution produced by the above method, and the significant compounds with an inclusive score of 70 or higher on the mzCloud Best Match are shown in Fig. 1.
Fig. 1.
(A) Main compounds in BST are indicated by < mzCloud Best Match score>, retention time/min, [molecular weight], (chemical formula), and name. (B) Red and black denote the total ion current diagram in negative and positive ion modes, respectively.
2.2. Experimental methods for model animals
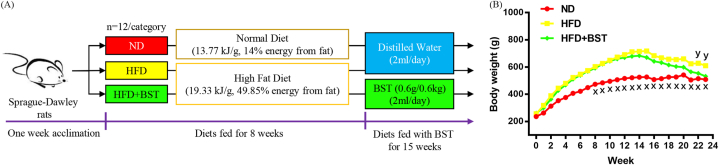
The Tongji Medical College Laboratory Animal Center, Huazhong University of Science and Technology (Wuhan, China) provided Sprague-Dawley rats (220–280 g, male, 8-week-old) and experimental diets (Table 1). Pathogen-free experimental conditions were used for animals with two rats per cage placed in a room with 22 2 °C temperature, 60 10% humidity, and 12/12 h light-dark cycle. Rats could freely access water and food, clinical signs were checked daily, and food intake and BW were evaluated weekly. Following acclimation for one week, rats were subjected to random separation into three categories, and each category had 12 rats (n 12): Animals were fed (1) a normal diet (13.77 kJ/g, 14% energy from fat) (ND), (2) HFD (19.33 kJ/g, 49.85% energy from fat) and (3) HFD BST. At week 8, an obesity model was successfully established, the HFD and HFD BST. The dosage was calculated according to the mean BW of the categories 600 g (one-sample t-test and P 0.05) shown in Table 3; the HFD BST received BST (0.6 g/0.6 kg) orally, while the ND and HFD received the same amount (2 ml) of distilled water orally.
Table 1.
Ingredients and energy composition of a normal diet and HFD.
| Normal Diet | High Fat Diet (HFD) | ||
|---|---|---|---|
| Barley flour (g/100 g) | 20 | Normal Diet (g/100 g) | 60 |
| Dehydrated vegetable (g/100 g) | 10 | Lard (g/100 g) | 17 |
| Soybean flour (g/100 g) | 20 | Yolk powder (g/100 g) | 10 |
| Yeast (g/100 g) | 1 | Skim milk powder (g/100 g) | 8 |
| Bone meal (g/100 g) | 5 | Casein (g/100 g) | 5 |
| Corn starch (g/100 g) | 16 | ||
| Bran (g/100 g) | 16 | ||
| Fish meal (g/100 g) | 10 | ||
| Salt (g/100 g) | 2 | ||
| Energy (kJ/g) | 13.77 | Energy (kJ/g) | 19.33 |
| Carbohydrate (kJ%) | 64.00 | Carbohydrate (kJ%) | 30.15 |
| Protein (kJ%) | 22.00 | Protein (kJ%) | 20.00 |
| Fat (kJ%) | 14.00 | Fat (kJ%) | 49.85 |
Table 3.
The body parameters of rats in each category are shown. Values were means SD (n 12), multi-group comparison by One-Way ANOVA; # (P 0.05), ## (P 0.01), and ### (P 0.001) vs. ND, and * (P 0.05), ** (P 0.01), *** (P 0.001) vs. HFD; a, P 0.05 vs. 600 g by one-sample t-test; b, P 0.05 by independent-samples t-test.
| ND | HFD | HFD BST | ||||
|---|---|---|---|---|---|---|
| Week 8 BW (g) | 474.17 | 54.41 *** | 603.48 | 72.18 ### a | 592.50 | 69.73 ### a |
| Final BW (g) | 509.42 | 57.89 *** | 610.40 | 73.28 ### | 532.70 | 75.21 ** |
| BW gain (g) | 35.25 | 20.07 | 6.92 | 42.52 | 59.80 | 43.06 ### *** |
| Week 8 BW 600 g | Week 8 BW 600 g | |||||
| 57.43 52.45 (n 7) b | 63.12 30.82 (n 5) b | |||||
| WC (cm) | 22.38 | 1.78 ** | 24.73 | 2.23 ## | 22.79 | 1.45 * |
| BMI (kg/m2) | 6.59 | 0.80 | 7.02 | 0.69 | 6.39 | 0.75 * |
| MFP (g) | 7.42 | 2.06 * | 9.61 | 2.40 # | 7.10 | 1.83 ** |
| PFP (g) | 10.11 | 3.32 *** | 21.42 | 7.98 ### | 13.95 | 5.55 ** |
| EFP (g) | 8.25 | 2.59 *** | 13.47 | 2.58 ### | 10.84 | 2.66 # * |
| Liver (g) | 13.91 | 2.18 | 15.37 | 2.41 | 13.51 | 2.30 |
Before euthanizing rats at week 23, the following parameters were evaluated; body mass index (BMI) as final BW/length2 (kg/m2), BW gain as final BW week 8 BW, and waist circumference (WC). Rats were fasted for 12 h before blood collection. The collected blood samples were placed at room temperature for 30 min, centrifuged at 3000 rpm for 10 min at 4 °C to obtain serum, and stored at 80 °C. Liver and fat pads of mesenteric (MFP), perirenal (PFP), and epididymal (EFP) regions were quickly removed and weighed. Liver fragments were either fixed in a 10% formalin solution or stored at 80 °C for further analyses. The experimental scheme is depicted in Fig. 2A. All experimental protocols were conducted in accordance with the Care and Use of Laboratory Animals guidelines set forth by the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee (IACUC 2788) of Tongji Medical College, Huazhong University of Science and Technology (Wuhan, China).
Fig. 2.
(A) Experimental scheme and (B) BW changes of rats in each category. Values were obtained by One-Way ANOVA multi-group comparison and denoted as means (n 12); x, P 0.001, ND vs. HFD; y, P 0.019, HFD BST vs. HFD.
2.3. Biochemical parameters measurement
Glucose concentration, insulin, ALT, TG, total cholesterol (TC), high-density lipoprotein cholesterol (HDLC), low-density lipoprotein cholesterol (LDLC), and C-reactive protein (CRP), were detected via enzymatic colorimetric kits (Nanjing Jiancheng Biotechnology Institute, Nanjing, China) following with manufacturer-recommended protocol. Measurement of insulin resistance was evaluated by homeostasis model assessment for insulin resistance (HOMA-IR) as fasting blood insulin (FIN; mU/L) fasting blood glucose (FBG; mmol/L)/22.5 [14].
2.4. Liver histopathological analysis
Fixed liver specimens were subjected to dehydration, embedding with paraffin, thin-section, and hematoxylin/eosin (HE) staining. Imaging was performed with acquirement by an upright microscope (OLYMPUS BX53, Tokyo, Japan).
2.5. Analysis of quantitative real-time polymerase chain reaction (qRT-PCR)
RNAiso Plus reagent (Takara, Japan) was used for liver RNA purification and ABScript II RT Master Mix (ABclonal, China) was used to synthesize cDNA. ChamQ Universal SYBR qPCR Master Mix (Vazyme Biotech Company, Nanjing, China) was utilized for qRT-PCR using a Fast Real-Time PCR System (7900HT, Thermos Fisher, USA). Table 2 lists the sequences for the primers. Gene expression was evaluated for relative gene expression levels of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal control by using the 2−ΔΔCt method.
Table 2.
Sequences of primers used for qRT-PCR.
| Genes | Forward (5’-3’) | Reverse (5’-3’) |
|---|---|---|
| SREBP1c | GTGACTTCCCTGGCCTATTTG | GCACGGACGGGTACATCTTT |
| ACC | CCCTGGAGTGGCAGTGGT | CCTTCACATAGCCTTTCTCATACA |
| FAS | TAATCCAGGGTCTCAGAAAAGC | TTTGGTGCCCGTCATAGGT |
| CPT1α | AAGGCTGCATAGCTGGACAA | CTGACTGGGTGGGATTAGAAGA |
| PPARα | GTGGCTGCTATAATTTGCTGTG | TTTGAAGGAGTTTTGGGAAGAG |
| AMPK | GCTGAGAAGCAGAAGCACGAC | CCAACAACATCTAAACTGCGAAT |
| GAPDH | CGCTAACATCAAATGGGGTG | TTGCTGACAATCTTGAGGGAG |
2.6. Analysis of statistics
Statistical analysis was performed using IBM SPSS Statistics Version 26 software (IBM Co., USA), and data were expressed as mean standard deviation (SD). Figures were created using GraphPad Prism Version 7 software (GraphPad, USA). Analysis of variance (ANOVA) was employed for multi-group comparisons, while the independent-samples t-test was used for two-group comparisons. Statistical significance was indicated when P 0.05, P 0.01, or P 0.001, denoted as #, ##, ### compared with ND, and *, **, *** compared with HFD, respectively.
3. Results
3.1. BST reduced HFD-induced BW gain, abdominal fat, and food intake
To evaluate the anti-obesity effects of BST, the BW of each category in this experiment was monitored weekly (Fig. 2B). At week 8, a well-established obesity model was observed in the HFD and HFD BST, with both showing a 20% increase in BW compared with the ND, P 0.001. No significant difference was observed between the HFD and HFD BST, P 0.05. From week 8–23, the HFD consistently exhibited higher BW compared with the ND, P 0.001, and BW loss in the HFD BST, P 0.019, appeared at week 22 and 23 (Fig. 2B). The HFD had increased final BW, P 0.001; and WC, P 0.004, compared with the ND. The HFD BST had decreased BW, P 0.010; WC, P 0.015; and BMI, P 0.044, at week 23, compared with the HFD. There was no significant difference in BW gain and BMI between the HFD and ND, with P 0.05 for both. However, the HFD BST exhibited a reduced BW gain compared with the ND and HFD, with P 0.001 for both comparisons. When assessed by wet weight, the HFD demonstrated increased MFP, P 0.016; PFP, P 0.001; and EFP, P 0.001, compared with the ND; the HFD BST showed decreased MFP, P 0.006; PFP, P 0.004; and EFP, P 0.019, compared with the HFD. The physical parameters of the rats are shown in Table 3. The ND had increased food intake compared with the HFD, P 0.023, and HFD BST, P 0.001, while the HFD BST, P 0.011, had decreased food intake, compared with the HFD. Energy intake was up-regulated in the HFD compared with the ND, P 0.001, and HFD BST, P 0.004. The rats’ food intakes are shown in Table 4. All rats showed typical clinical signs in the experiment.
Table 4.
Food and energy intake for each category. Values were means SD (n 24), One-Way ANOVA was used for multi-group comparisons; # (P 0.05), ### (P 0.001) vs. ND, and * (P 0.05), ** (P 0.01), *** (P 0.001) vs. HFD.
| ND | HFD | HFD BST | ||||
|---|---|---|---|---|---|---|
| Food intake/category (g) | 300.88 | 58.74 * | 266.03 | 51.06 # | 227.04 | 44.34 ### * |
| Energy intake/category (kJ) | 4143.07 | 808.84 *** | 5071.84 | 991.83 ### | 4316.41 | 782.36 ** |
3.2. BST reduced HFD-induced hyperlipidemia, insulin resistance, and inflammation
The HFD had increased serum levels of LDLC, TG, and TC, P 0.001 for all, compared with the ND. Serum levels of LDLC, P 0.001; TG, P 0.001; and TC, P 0.007, were decreased in the HFD + BST, compared with the HFD. The serum levels of HDLC were not markedly different P 0.05 among the ND, HFD, and HFD + BST. The HFD had increased FBG, FIN, and HOMA-IR, P 0.001 for all, compared with the ND. The HFD + BST had decreased FBG, P 0.001; FIN, P 0.007; and HOMA-IR, P 0.001, compared with the HFD. CRP serum levels were increased in the HFD, P 0.001, compared with the ND, and decreased in the HFD + BST, P 0.001, compared with the HFD. The biochemical parameters of the rats are shown in Table 5.
Table 5.
The biochemical parameters of rats in each category were shown. Values were denoted as means SD (n 12), One-Way ANOVA was used for multi-group comparison; # (P 0.05), ## (P 0.01), and ### (P 0.001) vs. ND, and * (P 0.05), ** (P 0.01), *** (P 0.001) vs. HFD.
| ND | HFD | HFD BST | ||||
|---|---|---|---|---|---|---|
| Serum TC (mmol/L) | 4.06 | 0.86 *** | 6.27 | 2.07 ### | 4.66 | 0.79 ** |
| Serum TG (mmol/L) | 1.92 | 0.39 *** | 3.24 | 0.56 ### | 2.35 | 0.42 # *** |
| Serum LDLC (mmol/L) | 0.74 | 0.14 *** | 1.77 | 0.26 ### | 1.04 | 0.15 ### *** |
| Serum HDLC (mmol/L) | 2.65 | 0.71 | 2.38 | 0.40 | 2.85 | 0.54 |
| FBG (mmol/L) | 6.63 | 0.93 *** | 10.27 | 1.93 ### | 8.25 | 0.81 ## *** |
| FIN (mU/L) | 11.57 | 1.09 *** | 15.83 | 3.12 ### | 13.28 | 1.78 ** |
| HOMA-IR | 3.43 | 0.76 *** | 7.41 | 2.74 ### | 4.92 | 1.06 # *** |
| Serum CRP (μg/L) | 1252.72 | 201.11 *** | 1684.57 | 347.27 ### | 1159.38 | 119.70 *** |
| Serum ALT (U/L) | 14.68 | 7.10 ** | 25.28 | 13.01 ## | 15.08 | 7.14 * |
| Hepatic TC (mmol/g) | 0.54 | 0.10 | 0.63 | 0.17 | 0.63 | 0.12 |
| Hepatic TG (mmol/g) | 1.46 | 0.57 *** | 2.56 | 0.79 ### | 1.60 | 0.72 ** |
3.3. BST reduced HFD-induced hepatic lipidosis
Biochemical parameters and liver histopathology were analyzed to identify the BST effect on reducing HFD-induced hepatic lipidosis. Liver weights did not show marked differences among the ND, HFD, and HFD BST, P 0.05 for all (Table 3). Serum ALT levels were increased in the HFD, P 0.010, compared with ND and decreased in the HFD BST, P 0.013, compared with HFD. The content of hepatic TC was not significantly different among the ND, HFD, and HFD BST, with P 0.05 for all. The content of hepatic TG was increased in the HFD, P 0.001, compared with ND and decreased in the HFD BST, P 0.002, compared with HFD (Table 5). Histological sections for livers with HE staining showed more vacuoles in the HFD compared with the ND and fewer in the HFD BST compared with the HFD (Fig. 3).
Fig. 3.
BST inhibited HFD-induced hepatic lipidosis. Liver HE staining in the (A) ND, (B) HFD, and (C) HFD BST (200 , vacuoles indicated by arrows).
3.4. Liver BST promoted oxidation of fatty acid and suppressed de novo lipogenesis
To elucidate the molecular mechanism of BST’s amelioration of hepatic lipidosis, qRT-PCR showed the gene expression for lipid metabolism. SREBP1c gene expression was increased in the HFD,1.37 0.46, P 0.005, compared with the ND, 1.00 0.13, and decreased in the HFD BST, 0.61 0.19, compared with the ND, P 0.003, and HFD, P 0.001 (Fig. 4A). ACC gene expression was increased in the HFD, 2.14 0.68, P 0.001, compared with the ND, 1.00 0.07, and decreased in the HFD BST, 0.73 0.19, P 0.001, compared with the HFD (Fig. 4B). In FAS gene expression, the HFD, 1.22 0.37, P 0.048, was increased compared with the ND, 1.00 0.07, no significant difference was shown between the HFD BST, 1.11 0.24 and the HFD, P 0.05 (Fig. 4C). In gene expression of CPT1α, the HFD, 0.72 0.20, P 0.002, was decreased compared with the ND, 1.00 0.12, and the HFD BST, 1.82 0.25 was increased compared with the HFD, and ND, P 0.001 for both (Fig. 4D). Gene expression of PPARα did not show a marked difference, P 0.05, between the HFD, 1.16 0.18, and ND, 1.00 0.08, while the HFD BST, 2.12 0.36 was increased than the HFD, and ND, P 0.001 for both (Fig. 4E). Expression of AMPK gene was not markedly different, P 0.05, between the HFD, 1.12 0.14, and ND, 1.00 0.12, while it was increased in the HFD BST, 1.77 0.34, compared with the HFD, and ND, P 0.001 for both (Fig. 4F).
Fig. 4.
BST promoted fatty acid oxidation and inhibited de novo lipogenesis in the liver. In each category, (A) SREBP1c, (B) ACC, (C) FAS, (D) CPT1α, (E) PPARα, and (F) AMPK gene expression. Values were denoted as mean SD (n 12) (with GAPDH as control), multi-group comparison by One-way ANOVA. P 0.05, P 0.01, and P 0.001 were denoted as #, ##, ### vs. ND, and *, **, *** vs. HFD, respectively.
4. Discussion
As the prevalence of obesity increases, there is growing interest in the effects and underlying mechanisms of BST on weight loss. Our findings revealed that BST contains a variety of polyphenols and flavonoids with anti-obesity properties (Fig. 1). For example, epigallocatechin, and catechin reduced BW, serum levels of LDLC, TG, TC, and enhanced tolerance to glucose and insulin in mice feeding HFD. Epigallocatechin, and catechin also decreased de novo lipogenesis and accumulation of lipids in the liver by increasing ACC and AMPK phosphorylation and down-regulating SREBP1c and FAS [31]. Myricetin reduced HFD-induced BW and abdominal fat mass, decreased LDLC, TC, and TG serum levels, and enhanced HDLC serum levels. Myricetin also reduced hepatic lipidosis by down-regulating SREBP1c, ACC, and FAS genes to suppress lipogenesis, and up-regulating the CPT1α gene to promote fatty acid oxidation [32]. Quercetin reduced serum glucose levels and hepatic lipid accumulation in mice induced by HFD [33]. Kaempferol inhibited BW gain, visceral fat accumulation, and hyperlipidemia in rats feeding HFD. Kaempferol also decreased hepatic TC and TG content through the down-regulation of SREBPs and up-regulation of PPARα [34]. The benefits of BST were probably associated with its components. Nonetheless, BST is a complex mixture, making it challenging to calculate the dosage of each component administered to each experimental subject and to determine the dose-effect relationship. Consequently, future studies should investigate the therapeutic effects of different ingredients in vitro and in vivo, the synergistic, additive, or antagonistic interactions among ingredient combinations, and the most effective therapeutic combinations. Moreover, extensive observations are needed to establish the correlation between the therapeutic effects of BST in animal models and humans to further validate the therapeutic outcomes of BST on human obesity and related metabolic disorders. This includes determining the effective dose of BST for individuals and the time required to yield positive results.
Previous research has demonstrated that isorhamnetin [35], quercetin [36], and genistein [37] contributed to reduce food and energy intake. However, other studies have indicated that the benefits of genistein [38], quercetin [39], and isorhamnetin and kaempferol [40] supplementation were independent of food and energy intake. Notably, BST was found to decrease food and energy intake in HFD-fed rats. Regrettably, the current investigations did not evaluate the underlying mechanism of BST′s effect on appetite in HFD-fed rats. It can be further explored in future studies.
Our experiment aimed to demonstrate that rats with obesity of distinct BW can lose weight with the same dose of BST. Consequently, rats in the HFD BST were orally administered the same BST dose (0.6 g) from the beginning of the treatment until the experiment′s conclusion. The difference in dosing according to this method and each rat′s BW could be attributed to rats weighing 0.6 kg receiving an increased BST dose and rats weighing 0.6 kg receiving a decreased BST dose. However, when rats in the HFD BST were classified by BW at week 8, there was no significant difference, P 0.05, in weight loss between rats weighing 0.6 kg, and those weighing 0.6 kg (Table 3), indicating that rats with different BWs could achieve the same degree of weight loss after receiving the same dose of BST treatment. In addition, the product-to-water ratio and tea concentration highly depend on the consumer′s experience and preferences. We can only confirm that each rat received the same gavage volume in this experiment.
The application of most dietary polyphenols and flavonoids in disease prevention is limited by their low water solubility, poor absorption, rapid metabolism, and unstable storage. Techniques such as food processing (mechanical, thermal, and non-thermal treatments), oral nanoformulations, enzymatic treatment, and co-culture with microorganisms and their metabolites can enhance bioavailability [41,42]. BST is a teabag product typically consumed using traditional extraction methods. Future studies could explore whether employing the abovementioned methods can enhance the variety, content, and bioavailability of bioactive components in BST. However, it is crucial to consider the cost-effectiveness of these processing approaches.
In conclusion, we have provided evidence that BST, containing various polyphenols and flavonoids, can effectively reduce obesity induced by HFD and its metabolic disorders, such as hyperlipidemia, inflammation, and insulin resistance. Furthermore, BST reduces hepatic lipidosis by enhancing fatty acid oxidation and suppressing de novo lipogenesis. Our data provide evidence supporting the possible health-beneficial effects of BST in the management of metabolic disorders and obesity.
Funding
This funding was granted by the National Natural Science Foundation of China (Grant No. 81873518).
Author contribution statement
Chingwen Yu, Xiaoning Wan: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper. Dan Li: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper. Xiaomei Guo: Conceived and designed the experiments; Wrote the paper. Data availability statement:Data will be made available on request. Declaration of interest’s statement: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We want to thank the researchers for their suggestions, support, and assistance in preparing this draft.
Abbreviations
- ACC
acetyl-coenzyme A carboxylase
- ALT
alanine transaminase
- AMPK
AMP-activated protein kinase
- ANOVA
analysis of variance
- BMI
body mass index
- BST
Besunyen Slimming Tea
- BW
body weight
- CPT1α
carnitine palmitoyltransferase1α
- CRP
C-reactive protein
- EFP
epididymal fat pads
- FAS
fatty acid synthase
- FBG
fasting blood glucose
- FIN
fasting blood insulin
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HDLC
high-density lipoprotein cholesterol
- HE
hematoxylin/eosin
- HFD
high fat diet
- HOMA-IR
homeostasis model assessment for insulin resistance
- LDLC
low-density lipoprotein cholesterol
- MFP
mesenteric fat pads
- NAFLD
nonalcoholic fatty liver disease
- PFP
perirenal fat pads
- PPARα
peroxisome proliferator-activated receptorα
- qRT-PCR
quantitative real-time polymerase chain reaction
- SD
standard deviation
- SREBP1c
sterol regulatory element binding protein1c
- TC
total cholesterol
- TG
triglyceride
- WC
waist circumference
References
- 1.Lin X., Li H. Obesity: epidemiology, pathophysiology, and therapeutics. Front. Endocrinol. 2021;12 doi: 10.3389/fendo.2021.706978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sirotkin A.V., Kolesárová A. The anti-obesity and health-promoting effects of tea and coffee. Physiol. Res. 2021;70(2):161–168. doi: 10.33549/physiolres.934674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sandoval V., Sanz-Lamora H., Arias G., Marrero P.F., Haro D., Relat J. Metabolic impact of flavonoids consumption in obesity: from central to peripheral. Nutrients. 2020;12(8):2393. doi: 10.3390/nu12082393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawser Hossain M., Abdal Dayem A., Han J., Yin Y., Kim K., Kumar Saha S., Yang G.M., Choi H.Y., Cho S.G. Molecular mechanisms of the anti-obesity and anti-diabetic properties of flavonoids. Int. J. Mol. Sci. 2016;17(4):569. doi: 10.3390/ijms17040569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang S., Moustaid-Moussa N., Chen L., Mo H., Shastri A., Su R., Bapat P., Kwun I., Shen C.L. Novel insights of dietary polyphenols and obesity. J. Nutr. Biochem. 2014;25(1):1–18. doi: 10.1016/j.jnutbio.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shin M.R., Shin S.H., Roh S.S. Diospyros kaki and citrus unshiu mixture improves disorders of lipid metabolism in nonalcoholic fatty liver disease. Chin. J. Gastroenterol. Hepatol. 2020 doi: 10.1155/2020/8812634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang T., Song J., Li J., Wang H., Zhang Y., Suo H. A synbiotic consisting of Lactobacillus plantarum S58 and hull-less barley β-glucan ameliorates lipid accumulation in mice fed with a high-fat diet by activating AMPK signaling and modulating the gut microbiota. Carbohydr. Polym. 2020;243 doi: 10.1016/j.carbpol.2020.116398. [DOI] [PubMed] [Google Scholar]
- 8.Choi D.J., Kim S.C., Park G.E., Choi B.R., Lee D.Y., Lee Y.S., Park S.B., Park Y.I., Kim G.S. Protective effect of a mixture of Astragalus membranaceus and lithospermum erythrorhizon extract against hepatic steatosis in high fat diet-induced nonalcoholic fatty liver disease mice. Evid. Based Complement Alternat. Med. 2020 doi: 10.1155/2020/8370698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inamdar S., Joshi A., Malik S., Boppana R., Ghaskadbi S. Vitexin alleviates non-alcoholic fatty liver disease by activating AMPK in high fat diet fed mice. Biochem. Biophys. Res. Commun. 2019;519(1):106–112. doi: 10.1016/j.bbrc.2019.08.139. [DOI] [PubMed] [Google Scholar]
- 10.Park M., Yoo J.H., Lee Y.S., Lee H.J. Lonicera caerulea extract attenuates non-alcoholic fatty liver disease in free fatty acid-induced HepG2 hepatocytes and in high fat diet-fed mice. Nutrients. 2019;11(3):494. doi: 10.3390/nu11030494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang H., Xie J., Wang N., Zhou Q., Lu Y., Qu Z., Wang H. Effects of Miao sour soup on hyperlipidemia in high-fat diet-induced obese rats via the AMPK signaling pathway. Food Sci. Nutr. 2021;9(8):4266–4277. doi: 10.1002/fsn3.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui S., Pan X.J., Ge C.L., Guo Y.T., Zhang P.F., Yan T.T., Zhou J.Y., He Q.X., Cheng L.H., Wang G.J., Hao H.P., Wang H. Silybin alleviates hepatic lipid accumulation in methionine-choline deficient diet-induced nonalcoholic fatty liver disease in mice via peroxisome proliferator-activated receptor α. Chin. J. Nat. Med. 2021;19(6):401–411. doi: 10.1016/S1875-5364(21)60039-0. [DOI] [PubMed] [Google Scholar]
- 13.Dusabimana T., Park E.J., Je J., Jeong K., Yun S.P., Kim H.J., Kim H., Park S.W. P2Y2R deficiency ameliorates hepatic steatosis by reducing lipogenesis and enhancing fatty acid β-oxidation through AMPK and PGC-1α induction in high-fat diet-fed mice. Int. J. Mol. Sci. 2021;22(11):5528. doi: 10.3390/ijms22115528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu D.X., Guo X.X., Zeng Z., Wang Y., Qiu J. Puerarin improves hepatic glucose and lipid homeostasis in vitro and in vivo by regulating the AMPK pathway. Food Funct. 2021;12(6):2726–2740. doi: 10.1039/d0fo02761h. [DOI] [PubMed] [Google Scholar]
- 15.Kim N.Y., Thomas S.S., Hwang D.I., Lee J.H., Kim K.A., Cha Y.S. Anti-obesity effects of morus alba L. And aronia melanocarpa in a high-fat diet-induced obese C57bl/6J mouse model. Foods. 2021;10(8):1914. doi: 10.3390/foods10081914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X., Yang L., Li J., Lin L., Zheng G. A flavonoid-rich Smilax China L. extract prevents obesity by upregulating the adiponectin-receptor/AMPK signalling pathway and modulating the gut microbiota in mice. Food Funct. 2021;12(13):5862–5875. doi: 10.1039/d1fo00282a. [DOI] [PubMed] [Google Scholar]
- 17.Shang A., Li J., Zhou D.D., Gan R.Y., Li H.B. Molecular mechanisms underlying health benefits of tea compounds. Free Radic. Biol. Med. 2021;172:181–200. doi: 10.1016/j.freeradbiomed.2021.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Neri-Numa I.A., Cazarin C.B.B., Ruiz A.L.T.G., Paulino B.N., Molina G., Pastore G.M. Targeting flavonoids on modulation of metabolic syndrome. J. Funct.Foods. 2020;73 doi: 10.1016/j.jff.2020.104132. [DOI] [Google Scholar]
- 19.Li D., Liu F., Wang X., Li X. Apple polyphenol extract alleviates high-fat-diet-induced hepatic steatosis in male C57bl/6 mice by targeting LKB1/AMPK pathway. J. Agric. Food Chem. 2019;67(44):12208–12218. doi: 10.1021/acs.jafc.9b05495. [DOI] [PubMed] [Google Scholar]
- 20.Khan N., Mukhtar H. Tea polyphenols in promotion of human health. Nutrients. 2018;11(1):39. doi: 10.3390/nu11010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez-Gonzalez S., Perez-Ramirez I.F., Amaya-Cruz D.M., Gallegos-Corona M.A., Ramos-Gomez M., Mora O., Reynoso-Camacho R. Polyphenol-rich peach (Prunus persica L.) by-product exerts a greater beneficial effect than dietary fiber-rich by-product on insulin resistance and hepatic steatosis in obese rats. J. Funct.Foods. 2018;45:58–66. doi: 10.1016/j.jff.2018.03.010. [DOI] [Google Scholar]
- 22.Tan Y., Kim J., Cheng J., Ong M., Lao W.G., Jin X.L., Lin Y.G., Xiao L., Zhu X.Q., Qu X.Q. Green tea polyphenols ameliorate non-alcoholic fatty liver disease through upregulating AMPK activation in high fat fed Zucker fatty rats. World J. Gastroenterol. 2017;23(21):3805–3814. doi: 10.3748/wjg.v23.i21.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santamarina A.B., Oliveira J.L., Silva F.P., Carnier J., Mennitti L.V., Santana A.A., de Souza G.H., Ribeiro E.B., Oller do Nascimento C.M., Lira F.S., Oyama L.M. Green tea extract rich in epigallocatechin-3-gallate prevents fatty liver by AMPK activation via LKB1 in mice fed a high-fat diet. PLoS One. 2015;10(11) doi: 10.1371/journal.pone.0141227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li B., Mao Q., Xiong R., Zhou D., Huang S., Saimaiti A., Shang A., Luo M., Li H., Li H., Li S. Preventive effects of different black and dark teas on obesity and non-alcoholic fatty liver disease and modulate gut microbiota in high-fat diet fed mice. Foods. 2022;11(21):3457. doi: 10.3390/foods11213457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Q., Lai X., Sun L., Cao J., Ling C., Zhang W., Xiang L., Chen R., Li D., Sun S. Antiobesity and anti-inflammation effects of Hakka stir-fried tea of different storage years on high-fat diet-induced obese mice model via activating the AMPK/ACC/CPT1 pathway. Food Nutr. Res. 2020:64. doi: 10.29219/fnr.v64.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Permatasari H.K., Nurkolis F., Gunawan W.B., Yusuf V.M., Yusuf M., Kusuma R.J., Sabrina N., Muharram F.R., Taslim N.A., Mayulu N., Batubara S.C., Samtiya M., Hardinsyah H., Tsopmo A. Modulation of gut microbiota and markers of metabolic syndrome in mice on cholesterol and fat enriched diet by butterfly pea flower kombucha. Curr. Res. Food Sci. 2022;5:1251–1265. doi: 10.1016/j.crfs.2022.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Permatasari H.K., Firani N.K., Prijadi B., Irnandi D.F., Riawan W., Yusuf M., Amar N., Chandra L.A., Yusuf V.M., Subali A.D., Nurkolis F. Kombucha drink enriched with sea grapes (Caulerpa racemosa) as potential functional beverage to contrast obesity: an in vivo and in vitro approach. Clin. Nutr. ESPEN. 2022;49:232–240. doi: 10.1016/j.clnesp.2022.04.015. [DOI] [PubMed] [Google Scholar]
- 28.Permatasari H.K., Nurkolis F., Augusta P.S., Mayulu N., Kuswari M., Taslim N.A., Wewengkang D.S., Batubara S.C., Ben Gunawan W. Kombucha tea from seagrapes (Caulerpa racemosa) potential as a functional anti-ageing food: in vitro and in vivo study. Heliyon. 2021;7(9) doi: 10.1016/j.heliyon.2021.e07944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li H.Y., Huang S.Y., Xiong R.G., Wu S.X., Zhou D.D., Saimaiti A., Luo M., Zhu H.L., Li H.B. Anti-obesity effect of theabrownin from dark tea in C57bl/6J mice fed a high-fat diet by metabolic profiles through gut microbiota using untargeted metabolomics. Foods. 2022;11(19):3000. doi: 10.3390/foods11193000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun Y., Wang Y., Song P., Wang H., Xu N., Wang Y., Zhang Z., Yue P., Gao X. Anti-obesity effects of instant fermented teas in vitro and in mice with high-fat-diet-induced obesity. Food Funct. 2019;10(6):3502–3513. doi: 10.1039/c9fo00162j. [DOI] [PubMed] [Google Scholar]
- 31.Li M., Xu J., Zhang Y., Chu S., Sun S., Huo Y., Zhao J., Hu X., Wan C., Li L. Comparative analysis of fecal metabolite profiles in HFD-induced obese mice after oral administration of huangjinya green tea extract. Food Chem. Toxicol. 2020;145 doi: 10.1016/j.fct.2020.111744. [DOI] [PubMed] [Google Scholar]
- 32.Xia S.F., Qiu Y.Y., Chen L.M., Jiang Y.Y., Huang W., Xie Z.X., Tang X., Sun J. Myricetin alleviated hepatic steatosis by acting on microRNA-146b/thyroid hormone receptor b pathway in high-fat diet fed C57BL/6J mice. Food Funct. 2019;10(3):1465–1477. doi: 10.1039/c8fo01452c. [DOI] [PubMed] [Google Scholar]
- 33.Snyder S.M., Zhao B., Luo T., Kaiser C., Cavender G., Hamilton-Reeves J., Sullivan D.K., Shay N.F. Consumption of quercetin and quercetin-containing apple and cherry extracts affects blood glucose concentration, hepatic metabolism, and gene expression patterns in obese C57bl/6J high fat-fed mice. J. Nutr. 2016;146(5):1001–1007. doi: 10.3945/jn.115.228817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang C.J., Tzeng T.F., Liou S.S., Chang Y.S., Liu I.M. Kaempferol regulates the lipid-profile in high-fat diet-fed rats through an increase in hepatic PPARα levels. Planta Med. 2011;77(17):1876–1882. doi: 10.1055/s-0031-1279992. [DOI] [PubMed] [Google Scholar]
- 35.Li J., Yuan H., Zhao Z., Li L., Li X., Zhu L., Wang X., Sun P., Xiao Y. The mitigative effect of isorhamnetin against type 2 diabetes via gut microbiota regulation in mice. Front. Nutr. 2022;9 doi: 10.3389/fnut.2022.1070908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tan Y., Tam C.C., Rolston M., Alves P., Chen L., Meng S., Hong H., Chang S.K.C., Yokoyama W. Quercetin ameliorates insulin resistance and restores gut microbiome in mice on high-fat diets. Antioxidants. 2021;10(8):1251. doi: 10.3390/antiox10081251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou L., Xiao X., Zhang Q., Zheng J., Li M., Yu M., Wang X., Deng M., Zhai X., Li R., Liu J. Dietary genistein could modulate hypothalamic circadian entrainment, reduce body weight, and improve glucose and lipid metabolism in female mice. Internet J. Endocrinol. 2019 doi: 10.1155/2019/2163838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li S., Zhou L., Zhang Q., Yu M., Xiao X. Genistein improves glucose metabolism and promotes adipose tissue browning through modulating gut microbiota in mice. Food Funct. 2022;13(22):11715–11732. doi: 10.1039/d2fo01973f. [DOI] [PubMed] [Google Scholar]
- 39.Chang W.L., Liu P.Y., Yeh S.L., Lee H.J. Effects of dried onion powder and quercetin on obesity-associated hepatic menifestation and retinopathy. Int. J. Mol. Sci. 2022;23(19) doi: 10.3390/ijms231911091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Othman Z.A., Wan Ghazali W.S., Noordin L., Mohd Yusof N.A., Mohamed M. Phenolic compounds and the anti-atherogenic effect of bee bread in high-fat diet-induced obese rats. Antioxidants. 2019;9(1):33. doi: 10.3390/antiox9010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Teng H., Zheng Y., Cao H., Huang Q., Xiao J., Chen L. Enhancement of bioavailability and bioactivity of diet-derived flavonoids by application of nanotechnology: a review. Crit. Rev. Food Sci. Nutr. 2023;63(3):378–393. doi: 10.1080/10408398.2021.1947772. [DOI] [PubMed] [Google Scholar]
- 42.Polia F., Pastor-Belda M., Martínez-Blázquez A., Horcajada M.N., Tomás-Barberán F.A., García-Villalba R. Technological and biotechnological processes to enhance the bioavailability of dietary (Poly)phenols in humans. J. Agric. Food Chem. 2022;70(7):2092–2107. doi: 10.1021/acs.jafc.1c07198. [DOI] [PMC free article] [PubMed] [Google Scholar]