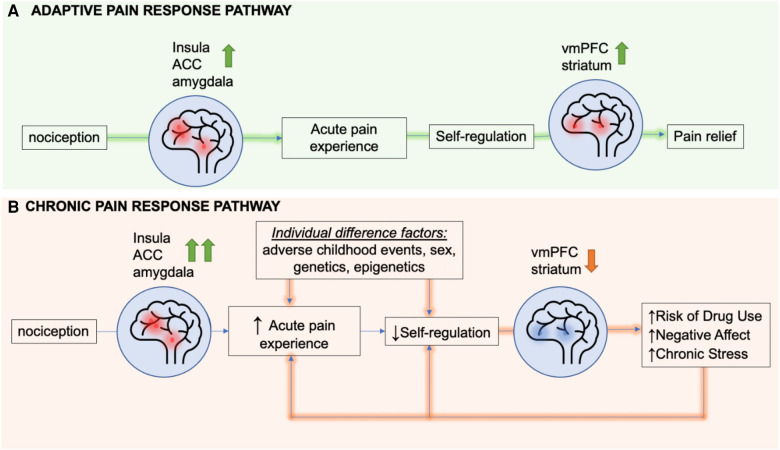
Figure 4.
A heuristic feed-forward model of overlapping stress circuits and circuits driving risk of chronic pain. Presentation of a noxious stimulus results in activation of the insula, amygdala, and dorsal anterior cingulate cortex (dACC). In the adaptive pain pathway, the ventromedial prefrontal cortex (VmPFC) and striatum engage soon after the start of the acute pain experience to regulate nociceptive signals, thereby mediating pain relief. In chronic pain, factors such as adverse childhood events, sex, genes, drug use, negative affect, and stress can all increase the activity of the insula, dACC and amgydala following noxious stimulus presentation and also result in hypoactivation of the VmPFC and striatum, identified as the resilient coping and self regulation circuit. This hypoactivation impairs self-regulation of not only stress but also pain states, thereby extending the pain experience. Both heightened pain sensitivity and longer lasting pain predispose patients to risk of drug use, negative affect, and chronic stress, that in turn creates a feed-forward sensitized pathway towards more hypoactivation of the coping and self regulation circuit, thereby increasing risk of sustained pain symptoms and chronic pain.