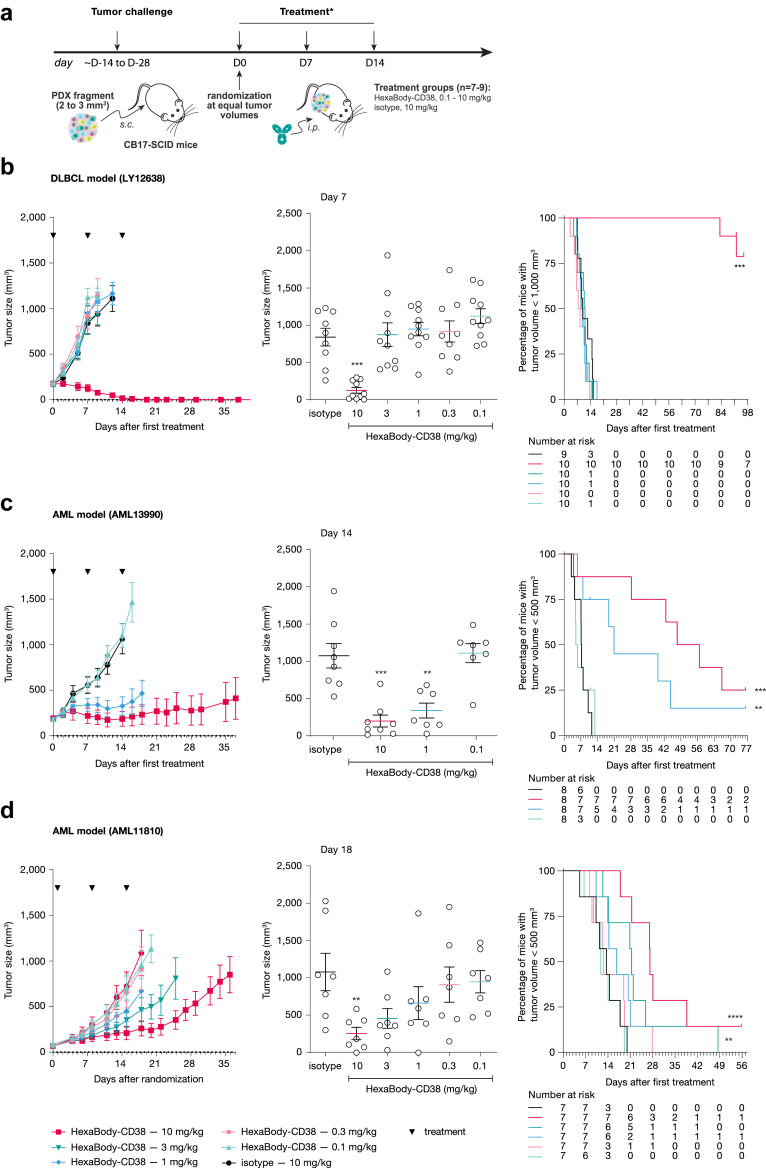
Fig. 5.
Evaluation of Dose-dependent anti-tumour activity of HexaBody-CD38 on established B-NHL and AML PDX tumours in vivo. (a) Experimental set up of the evaluation of the anti-tumour activity of HexaBody-CD38 in PDX models in vivo. ∗: treatment started 1 day after randomization in the AML11810 model. Dose-dependent anti-tumour activity of HexaBody-CD38 (3 weekly IV injections) was evaluated in (b) B-NHL PDX model Ly12638 and in AML PDX models (c) AML13990 and (d) AML11810. Treatment with isotype control antibody Hx-ctrl (10 mg/kg) was included as negative control. Left panels show mean tumour growth ± SEM for each treatment group in time. Middle panels show the tumour size for each individual mouse plotted per treatment on the last day that all groups were complete: Day 7 for model Ly12638 (b), Day 14 for model AML13990 (c), and Day 18 for model AML11810 (d). C). ∗∗P < 0.01, ∗∗∗P < 0.001; Mann Whitney test versus Hx-ctrl. Right panels show the % of mice with tumour sizes smaller than 1000 or 500 mm3, as indicated, in Kaplan–Meier plots. ∗∗P < 0.01, ∗∗∗P < 0.001, ∗∗∗∗P < 0.0005; Mantel Cox Analysis versus Hx-ctrl.