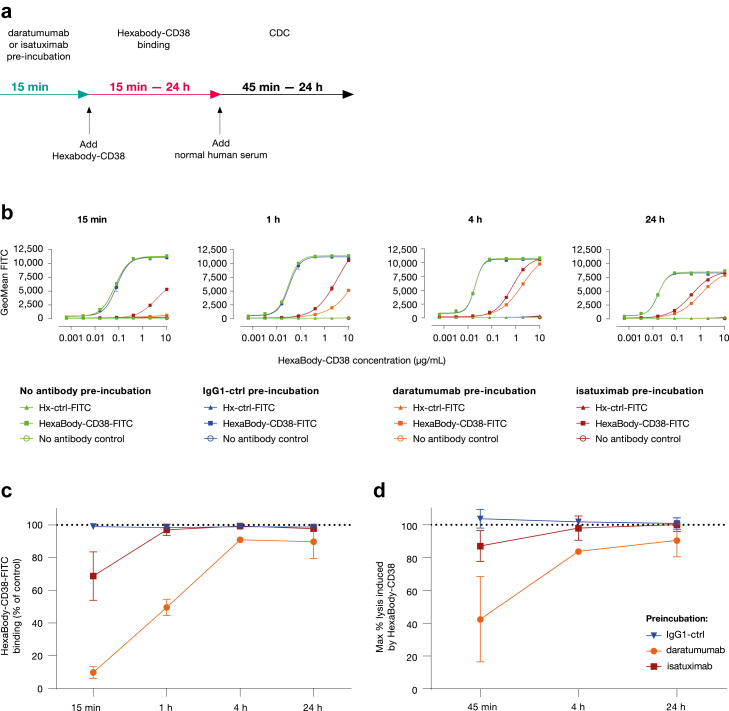
Fig. 7.
Binding and CDC induction of HexaBody-CD38 in the presence daratumumab or isatuximab. (a) Experimental set-up to determine the binding and CDC activity of HexaBody-CD38 on Wien-133 cells in the presence of daratumumab or isatuximab. (b) Dose-dependent binding of FITC-conjugated HexaBody-CD38 is shown over time in the presence of daratumumab or isatuximab at a saturating concentration (10 μg/mL). After 15 min incubation with a concentration range of HexaBody-CD38-FITC, the cells were either directly evaluated by flow cytometry (15 min) or incubated for an extended time to evaluate binding after 1 h, 4 h, or 24 h incubation. (Pre-) incubation with IgG1-ctrl or without antibody were included as negative controls. Fluorescence intensity is shown as a measure of antibody-binding. Representative experiments from at least three independent experiments are shown. (c) HexaBody-CD38 (10 μg/mL) binding in the presence of IgG1-ctrl, daratumumab, or isatuximab over time as percentage of HexaBody-CD38 binding without antibody pre-incubation. (d) The capacity of HexaBody-CD38 (10 μg/mL) to induce CDC of Wien-133 cells in the presence of equal amounts of daratumumab or isatuximab over time. CDC was evaluated by flow cytometry after 45 min, 4 h, or 24 h. (Pre-) incubation with IgG1-ctrl, or without antibody were included as negative controls. The % lysis was calculated as 100% minus the % TO-PRO-3-negative (viable) cells. Mean ± SD of antibody binding (c) and lysis (d) are shown from at least three independent experiments (except for the 4 h timepoint in the presence of daratumumab, which was only included once).