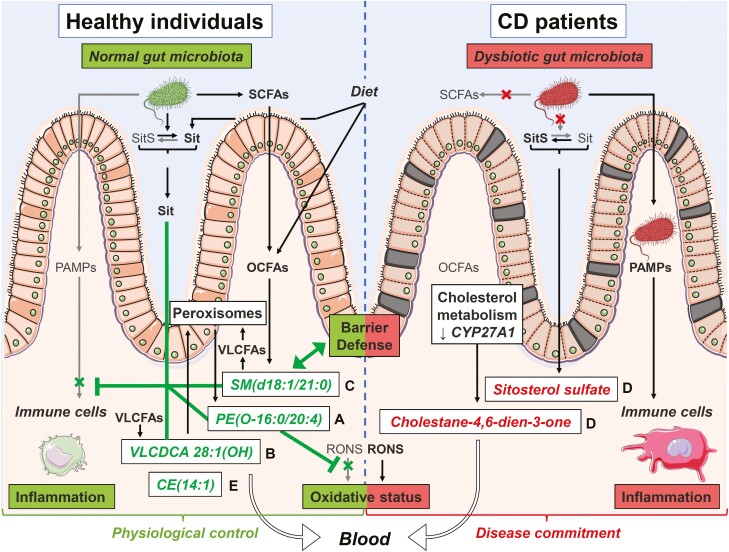
Figure 6.
Putative mechanisms underlying observed changes in lipid candidate biomarkers and their impact on Crohn’s disease (CD) pathophysiology. This figure illustrates the proposed mechanisms underlying the observed changes (lower in green, higher in red) in serum levels of candidate biomarker lipids and their correlated clusters (letters on the right of lipid names) in CD patients. We reason that these changes may restrict (┬) or stimulate (↑) various biological functions and mechanisms linked to the pathogenesis of CD. Lipids from clusters with lower serum levels in CD vs control (clusters A-C and E) will be unable to carry out their normal physiological roles, notably (1) restricting inflammation either by preventing its overtriggering by pathogen-associated molecular patterns (PAMPs) or by favoring its resolution through specialized proresolution mediator–like mechanisms (clusters A and B), (2) strengthening the defense barrier of the intestinal mucosa (cluster C), and/or (3) maintaining a physiological oxidative stress status by restricting reactive oxygen and nitrogen species (RONS) effects (cluster A). Furthermore, in CD, dysbiosis is expected to impair metabolism of (1) short-chain fatty acids (SCFAs) and ultimately that of odd-chain fatty acids (OCFAs), which are normally incorporated into sphingomyelins (SMs) (cluster C) and (2) noncholesterol sterols resulting in sitosterol (Sit) being converted into sitosterol sulfate (SitS) (cluster D). Dysregulated host metabolism involving peroxisomes or cytochrome P450 enzymes is also expected to impair formation of polyunsaturated fatty acid (PUFA)–containing ether lipids (cluster A), very long-chain dicarboxylic acids (VLCDCAs) (cluster B), very long-chain fatty acid (VLCFA)–containing SMs (cluster C), and cholesterol esters (CEs) (cluster E), while favoring the formation of a specific oxysterol, namely cholesta-4,6-dien-3-one (cluster D). Enhanced oxidative stress in CD may also contribute to lower serum levels of PUFA-containing ether lipids (cluster A), while the defective intestinal barrier function may result in serum over-representation of SitS and cholesta-4,6-dien-3-one (cluster D). Altogether, these changes and others may contribute to the progression from a physiological control of crucial intestinal processes toward a commitment of CD. Figure created by Servier Medical Art (SMART) images by Servier (http://smart.servier.com/), licensed under a Creative Commons Attribution 3.0 Unported License (CC BY 3.0).