Abstract
Study objectives:
Healthy infants may have a greater apnea hypopnea index (AHI) than older children during the newborn period, but the trajectory of these sleep-related events beyond the first month of life is poorly understood. In this study, we evaluated the longitudinal changes in respiratory indices during sleep in healthy infants during the first six months of life.
Methods:
Single-center prospective cohort study. Thirty healthy infants underwent overnight in-lab polysomnography at one and five months of age and findings were compared between assessments. Systematic review of studies evaluating infant polysomnography and meta-analysis was conducted.
Results:
At one month of age, total AHI, obstructive AHI, and central AHI model-adjusted means (95% confidence interval) were 16.9 events/hour (12.2, 21.5), 10.2 events/hour (7.4, 13.1), and 6.6 events/hour (4.2, 9.0), respectively. 16.8% of events were obstructive apneas and 36.1% central apneas. By five months of age, there were significant reductions in each index to 4.1 events/hour (3.2, 5.0), 1.9 events/hour (1.4, 2.4), and 2.2 events/hour (1.6, 2.9), respectively (p<0.001 for each), and a lower proportion of events were obstructive apneas (8.6%, p=0.007) and a greater proportion central apneas (52.3%, p=0.002). Meta-analysis found high AHI in infants with significant heterogeneity.
Conclusions:
Central AHI and obstructive AHI are greater in healthy newborns than older children. There is a significant spontaneous reduction in events and change in type of events in the first six months of life in this low-risk population. These findings may serve as a reference for clinicians evaluating for obstructive sleep apnea in infants.
Keywords: obstructive sleep apnea, infant, newborn, polysomnography
1. Introduction
Polysomnography (PSG) is the gold standard for evaluation of obstructive sleep apnea (OSA) in children of all ages, from infancy through adolescence1. However, there are limited normative respiratory data in healthy infants, making the interpretation of OSA status in this age group fraught with challenges. Available data suggest that in comparison to older children, infants have increased obstructive, mixed, and central apneas within the first weeks of life, but studies are limited to abbreviated daytime tests, polygraphy studies that did not include encephalogram, and scoring that predated current guidelines2–4. Few African Americans were included in previous studies, which could potentially lead to an under-estimation of apnea hypopnea index (AHI) in the larger population5. Importantly, the trajectory of respiratory events during sleep beyond the first month of life in healthy infants is also poorly understood. Limited polygraphy data suggest that there are fewer obstructive events after only a few months of growth4, but no longitudinal data using PSG are available.
Multiple physiological differences may explain the propensity toward more respiratory events during sleep in infancy when using the current American Academy of Sleep Medicine pediatric scoring rules6. The upper airway in healthy infants, while resistant to complete collapse, is highly compliant, increasing its susceptibility to significant changes in cross-sectional area with small changes in luminal pressure, resulting in ventilatory instability and obstructive cycling7,8. A highly compliant rib cage results in paradoxical breathing during sleep9. Further, infants spend a greater proportion of total sleep time in rapid eye movement (REM) sleep than older children, where there is greater respiratory instability and more obstructive apnea10,11.Thus, there are multiple drivers of differences in PSG results that can evolve in the first months after birth.
Despite these uncertainties surrounding their interpretation, the use of PSG is increasing in high-risk infants being evaluated for OSA, including those with craniofacial conditions, Down syndrome, Prader-Willi syndrome, laryngomalacia, and others12–16. The paucity of normative data in these situations poses further challenges in medical decision-making, including when treatment with surgery and continuous positive airway pressure17–19 are indicated. A lack of consistent metrics, including comparison with normative data in this age group, has prevented the development of standardization in treatment regimens for these high-risk infants13 leading to inconsistent practice in the field20,21.
This study aimed to assess respiratory data during the newborn period in a diverse sample of healthy infants using in-laboratory, overnight PSG and to evaluate changes in PSG parameters in the first six months of life. In addition, the study aimed to conduct a systematic review of published studies evaluating respiratory indices during sleep in healthy infants using polysomnography for meta-analysis. We hypothesized that there would be more central and obstructive apneas in healthy newborns than older children and that there would be significant reductions in these obstructive and central apneas with only a few months of normal maturation in the healthy newborn population.
2. Materials and Methods:
2.1. Study design and participants
This was a single-center prospective longitudinal cohort study. A community-based sample of healthy newborns born at least 37 weeks’ gestation was recruited in the first two weeks of life between May 2016 and July 2019 from well-baby units or initial well visits with their pediatrician. Healthy infants did not have any previous history of cardiorespiratory or neurologic problems, had no history of previous illness or concerns about breathing during sleep, and no first-degree relative with OSA. The study was approved by the Children’s Hospital of Philadelphia Institutional Review Board (#14–011346). Informed consent was obtained from the parent of each participant in the study.
2.2. Assessments
Baseline visit was completed at one month of age and infants returned for a follow-up visit at five months of age to allow for several months of maturation with a second assessment to be completed by six months of age. Medical records were reviewed when available. Infants underwent medical history and physical exam to confirm that they were well and to specifically assess for visible evidence of hypotonia or craniofacial abnormality.
At both visits, in-lab overnight diagnostic PSG was conducted and scored using American Academy of Sleep Medicine criteria, including infant sleep staging for the baseline visit6. Because phases of NREM sleep (N1, N2, N3) could not be identified in all studies, especially at one month of age, only the proportion of REM was included in the final analysis. Participants reported to the sleep laboratory at 6:30 PM with a parent for setup and were studied until 7 AM the following morning. All infants were placed in the supine position for PSG. A Polysmith PSG system (Nihon Kohden, Irvine, CA) was used to record the following parameters: electroencephalography (leads at C3A2, C4A1, F3A2, F4A1, O1A2, O2A1); bilateral electrooculograms; submental and tibial electromyograms; chest and abdominal wall motion using respiratory inductance plethysmography (Natus, Middleton, WI); heart rate by electrocardiogram; arterial oxygen saturation by pulse oximetry (Masimo, Irvine, CA); end-tidal carbon dioxide measured at the nose by infrared capnometry (Novametrix Medical System, Inc., Wallingford, CT); airflow using a 3-pronged thermistor (Pro-Tech Services, Inc., Mukilteo, WA) and nasal pressure (Pro-Tech Services, Inc., Walnut Cove, NC). Participants were continuously observed by a PSG technician in a dark room and audio/video was recorded with the use of an infrared video camera and microphone.
2.3. Systematic review
The systematic literature review was conducted in accordance with the PRISMA-P protocol22. The search for peer-reviewed research articles, without language restriction or publication dates was performed (by DS) using the following search term for querying the PUBMED database:
((AHI[Title/Abstract]) OR (“apnea-hypopnea index”[Title/Abstract]) OR (“central apnea index”[Title/Abstract]) OR (“obstructive apnea index”[Title/Abstract]) OR (“sleep apnea”[Title/Abstract])) AND (infants[Title/Abstract]) NOT (Robin[Title/Abstract]) NOT(Review[Title/Abstract])
Manuscripts included in the final meta-analysis were selected using a selection carried out by two of the authors (CC and AC). If there was a disagreement, a third author (IET) was consulted. To be included, studies were required to include polysomnography of healthy infants less than 12 months of age and report respiratory outcomes associated with sleep-disordered breathing, including either AHI and/or sub-indices.
Information from the selected articles was curated by the authors (CC and AC) and stored in a Microsoft Excel (Microsoft, Seattle WA) spreadsheet. The primary outcomes considered were AHI, obstructive AHI (OAHI), and central apnea index (CAI); mixed apnea index (MAI), obstructive hypopnea index (OHI), and obstructive apnea index (OAI) were also considered. Secondary outcomes included oxyhemoglobin saturation nadir, mean oxyhemoglobin saturation, and proportion of total sleep time with saturation below 90%. Of note was that not all articles in our database had the full set of indices. When possible, if one of the above-mentioned indices was missing, it was derived from the data presented. When studies included multiple age cohorts, they were included separately in the analysis.
2.4. Data analysis
All analyses were conducted with Stata 16MP (StataCorp, College Station TX) with two-sided tests of hypotheses and a p-value < 0.05 as the criterion for statistical significance.
Analysis of the prospective cohort: Descriptive analyses included computation of medians and ranges of continuous variables and tabulation of categorical variables. Tests of normal distribution were performed to determine the extent of skewness of variables. Frequency counts and percentages were used to report categorical variables. Inference statistical analysis were conducted in two steps. Due to non-normality of most PSG variables, mixed-effects models were constructed. Univariate analysis was used to identify statistically significant confounders. The visit type (baseline at 1 month versus 4-month follow-up) was included as fixed effects in the model. Random effects were set on the level of individual patient. Mixed-effects models are capable of handling missing values as there were five participants who were not able to return for follow-up testing. To adjust for small departures from normality, robust (sandwich) estimation of the variance was used. Post-hoc assessment of the marginal (model adjusted) means and differences were estimated in pairwise fashion. Least significant mean method was used to adjust for multiple comparisons. Marginal means and differences are reported with their respective 95% confidence interval unless otherwise specified.
Analysis for the systematic review: Random-effects meta-analysis was performed using restricted maximum likelihood method23. Random-effects model was used for all indices since early on a high level of heterogeneity was observed for most indices. When the mean and the standard deviation were not available, they were estimated from median and the ranges or interquartile ranges using computation proposed by Wan et al24. The heterogeneity of studies was assessed by the I2 statistics where values more than 50% were indicative of heterogeneous studies. Furthermore, Cochran’s Q test with P<0.1 was considered as indication of heterogeneity as well. The heterogeneity of the selected studies was also assessed using visual inspection of the Funnel plots25.
3. Results:
3.1. Primary data
Thirty healthy infants completed baseline PSG at one month of age. Demographics for participants are shown in Table 1.
Table 1.
Demographics.
| Parameter | Study cohort (n=30) |
|---|---|
| Age at baseline visit, weeks | 3.7 (2.0, 7.1) |
| Gestational age at birth, weeks | 39.6 (37, 41) |
| Early term (37–38 weeks), n % | 7 (23.3) |
| Race, n (%) | |
| Black | 15 (50) |
| White | 11 (36.7) |
| Asian | 0 |
| Other | 4 (13.3) |
| Ethnicity, Latinx, n (%) | 5 (16.7) |
| Sex, female, n (%) | 13 (43.3) |
Values are in median (95% CI) unless otherwise specified.
All infants were born at term, half of the participants were Black and there was a slight male predominance; Black race, White race, and Latinx ethnicity were similar to 2020 Philadelphia county demographics. Univariate analysis of potential confounders, including age at visit, gestational age, race (self-reported), ethnicity (self-reported), and sex, did not identify any significant confounders of primary or secondary outcomes. At one-month PSG, all healthy infants had a significant number of obstructive and central events (Table 2). The model-adjusted mean (95% CI) AHI was 16.9 events/hour (12.2, 21.5), obstructive AHI was 10.2 events/hour (7.4, 13.1) and central apnea index was 6.6 events/hour (4.2, 9.0).
Table 2:
Change in polysomnographic parameters from baseline to 4-month follow-up in healthy infants.
| Parameter | One month old (n=30) | Five months old (n=25) | p |
|---|---|---|---|
| Age, weeks | 3.8 (3.3, 4.4) | 22.4 (21.3, 23.5) | |
| AHI (n/hour) | 16.9 (12.2, 21.5) | 4.1 (3.2, 5.0) | <0.001 |
| Obstructive apnea hypopnea index, n/hour | 10.2 (7.4, 13.1) | 1.9 (1.4, 2.4) | <0.001 |
| Time in airway obstruction (%) | 1.9 (1.3, 2.4) | 0.4 (0.3, 0.6) | <0.001 |
| Obstructive apnea index, n/hour | 3.1 (1.6, 4.6) | 0.3 (0.2, 0.5) | <0.001 |
| Obstructive hypopnea index, n/hour | 5.4 (3.8, 6.9) | 1.3 (0.8, 1.8) | <0.001 |
| Mixed apnea index, n/hour | 1.8 (1.0, 2.6) | 0.3 (0.1, 0.4) | <0.001 |
| Central apnea index, n/hour | 6.6 (4.2, 9.0) | 2.2 (1.6, 2.9) | <0.001 |
| Periodic breathing, % total sleep time | 0.5 (0.2, 1.0) | 0.2 (0.1, 0.3) | 0.14 |
| SpO2 nadir, % | 85.5 (83.3, 87.6) | 87.2 (84.6, 89.7) | 0.31 |
| Desaturation nadir below 80%, n (%) | 4 (13.3) | 3 (12) | 0.89 |
| Mean SpO2, % | 97.9 (97.5, 98.4) | 98.6 (98.2, 99.0) | 0.002 |
| Total sleep time with SpO2<90%, % | 0.3 (0.1, 0.4) | 0.1 (0, 0.2)* | 0.016 |
| Maximum etCO2, mm Hg | 48.3 (46.9, 49.7) | 47.8 (46.2, 49.4) | 0.59 |
| Sleep time with etCO2>50 mm Hg, % | 0.2 (0, 0.4)* | 0.02 (0, 0.03)* | 0.08 |
| Sleep efficiency, % | 76.8 (73.7, 79.9) | 87.9 (86.1, 89.6) | <0.001 |
| REM sleep, % total sleep time | 45.6 (42.7, 48.9) | 37.3 (34.2, 40.3) | <0.001 |
| Arousal index, n/hour | 22.9 (19.9, 25.8) | 14.4 (12.6, 16.3) | <0.001 |
Summary polysomnography data from 30 healthy infants, 25 of whom completed both visits.
Results shown are model estimated adjusted mean and 95% confidence interval.
EtCO2, end-tidal CO2; SpO2, arterial oxygen saturation.
Model resulted in a negative number for the lower bound of the 95% CI and was unable to determine whether the marginal mean was different than zero.
Time in airway obstruction was 1.9% (1.3, 2.4) of total sleep time. Of the total respiratory events at baseline, an adjusted mean of 18% of events were obstructive apneas, with 39% central apneas, 32% obstructive hypopneas, and 11% mixed apneas. There was little associated desaturation or hypercapnia. No healthy infant had saturation below 90% for greater than 0.5% of their total sleep time. The sleep time with end-tidal CO2 greater than 50 mm Hg was 0.18% (0, 0.37) for the cohort. There were no significant differences in the PSG results (AHI, obstructive apnea index, obstructive hypopnea index, mixed apnea index, and central apnea index) based on sex, race, or ethnicity, and no significant associations with gestational age or age at baseline visit (all p>0.05).
Five healthy infants were lost to follow-up and twenty-five returned for repeat PSG at five months of age (Figure 1). None of the participants were admitted to the neonatal intensive care unit after birth or hospitalized prior to follow-up visit and none were treated for OSA or other respiratory conditions during the study. There was a significant reduction in AHI [4.1 events/hour (3.2, 5.0), p<0.001] as well as time in airway obstruction [0.4% (0.3, 0.6), p<0.001] compared to baseline testing (Figure 2). There were significant reductions in all the respiratory indices, including obstructive apnea index [0.3 events/hour (0.2, 0.5), p<0.001], obstructive hypopnea index [1.3 events/hour (0.8, 1.8), p<0.001], mixed apnea index [0.3event/hour (0.1, 0.4), p<0.001], and central apnea index [2.2 events/hour (1.6, 2.9), p<0.001]. The time spent in respiratory events decreased proportionally [0.4 (0.3, 0.6), p<0.001]. There was a significant change in the proportion of respiratory event types at the five-month-old visit with 8.6% (4.0, 13.2) obstructive apneas (p=0.007) and 52.3% (42.2, 62.3) central apneas (p=0.002) (Figure 2). Compared to baseline testing, by the 5-month visit, sleep efficiency had increased significantly, associated with reduced nocturnal feeding during the study. In addition, there was a lower proportion of REM sleep and fewer arousals at the 5-month visit compared to baseline.
Figure 1.
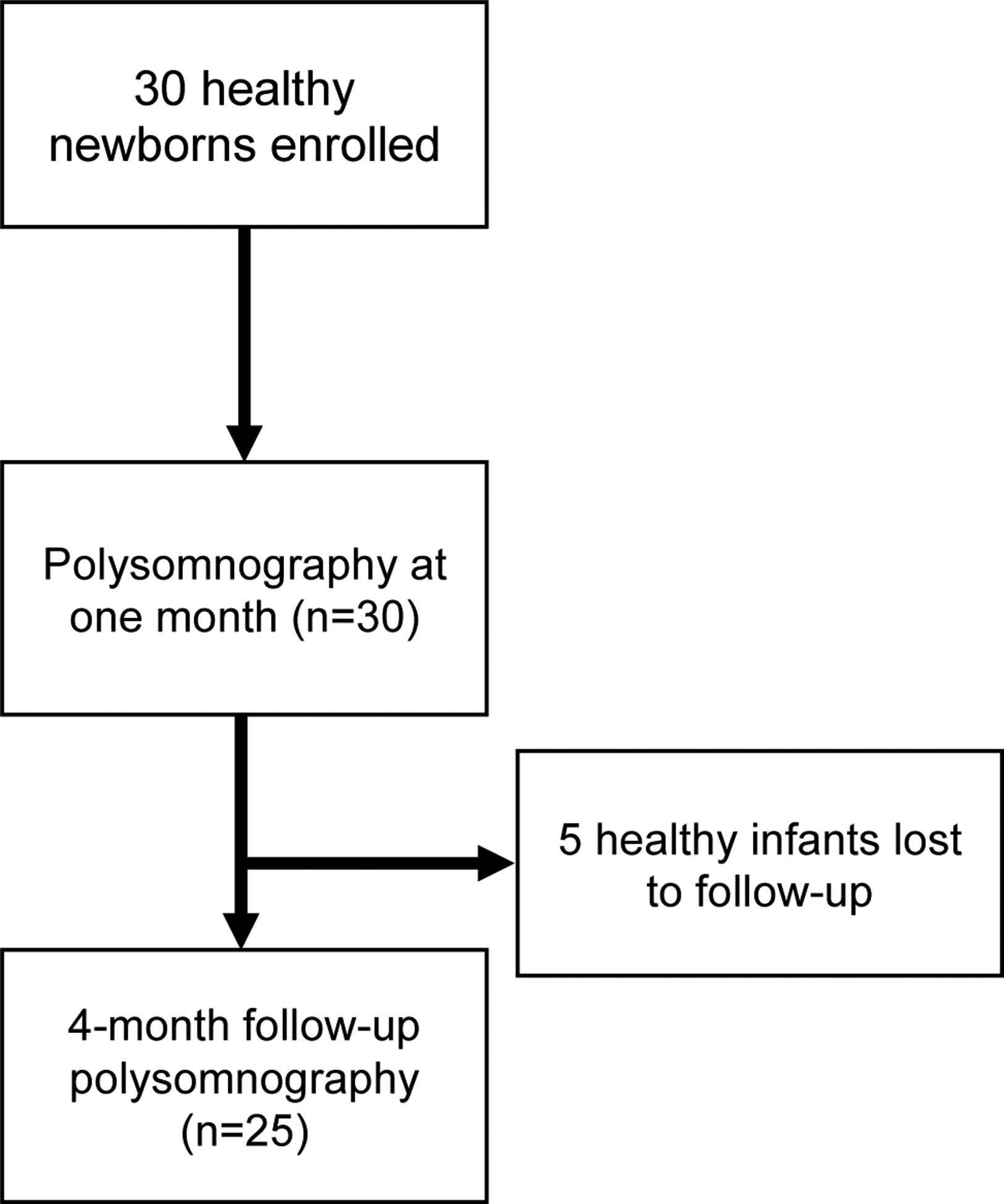
Consort diagram.
Figure 2.
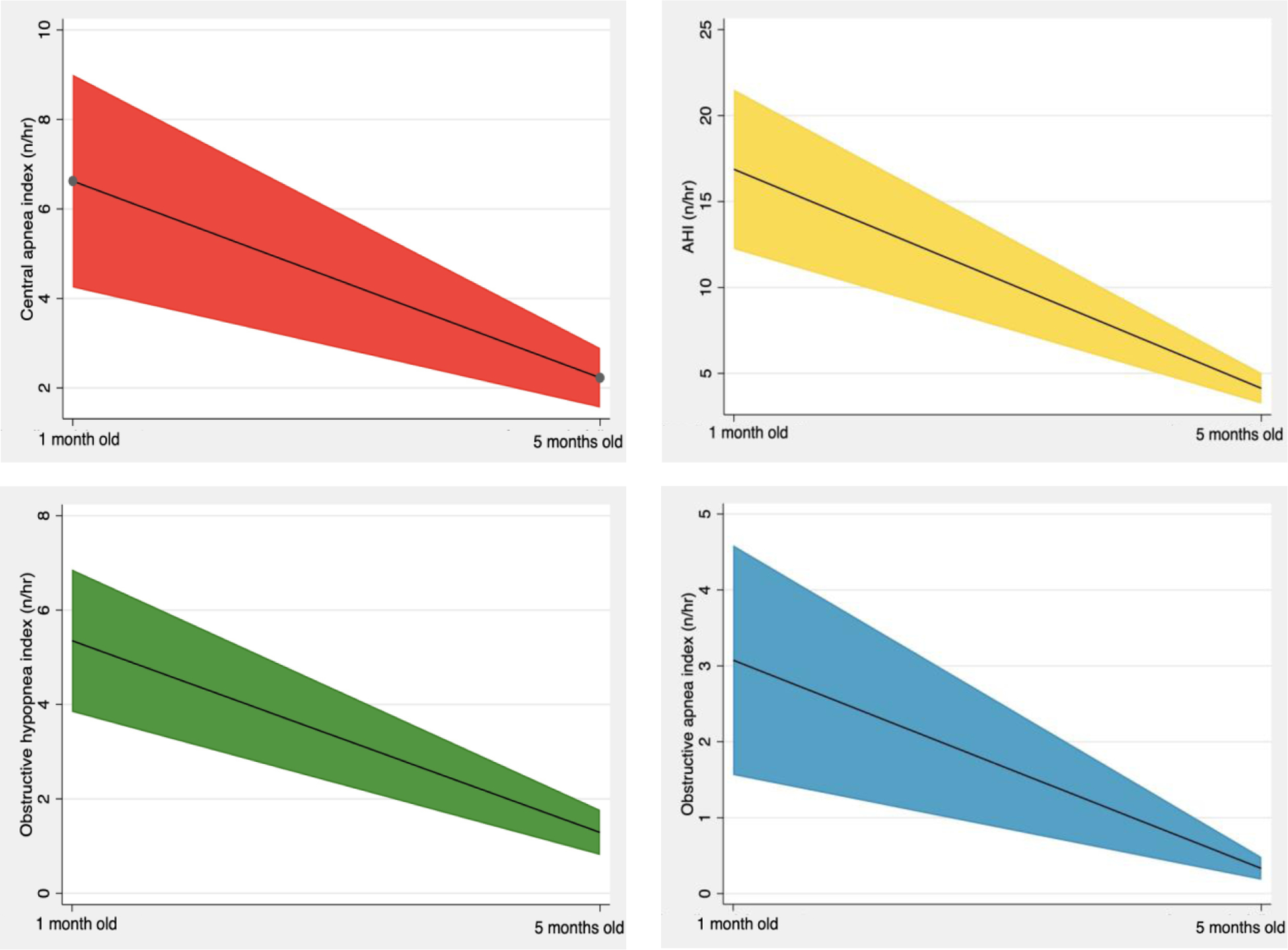
Change in respiratory polysomnographic indices from one month to five months of age. For each panel, black line represents model-adjusted mean and colored portion the 95% confidence interval.
3.2. Systematic Review
A total of 101 articles were identified satisfying the above-mentioned search terms. Except for one article emanating from our group26, the authors on any of the identified article were not contacted for any additional information.
Ten studies3,4,26–33 met inclusion criteria for meta-analysis out of a total of 101 articles (Figure 3), including one (Duenas-Meza 2022), which had two separate age cohorts that were both analyzed. This study assessed infants living at altitude (2640 meters above sea level) and was included for completeness. 59 articles were excluded because they did not include healthy infants, 8 articles were excluded because they only included participants younger than 12 months, 2 because the article was not available in English, 12 because the participants were not studied with polysomnography or polysomnography variables were not available, 4 because they were unrelated to the topic, and 6 because they were review articles. The year of publication varied from 1981 to 2022. The number of subjects per study varied from 7 to 400. Techniques varied, primarily as a function of the study date, including the sensors used in polysomnography and the scoring of events, but all studies included measures of events per hour as the primary outcome.
Figure 3.
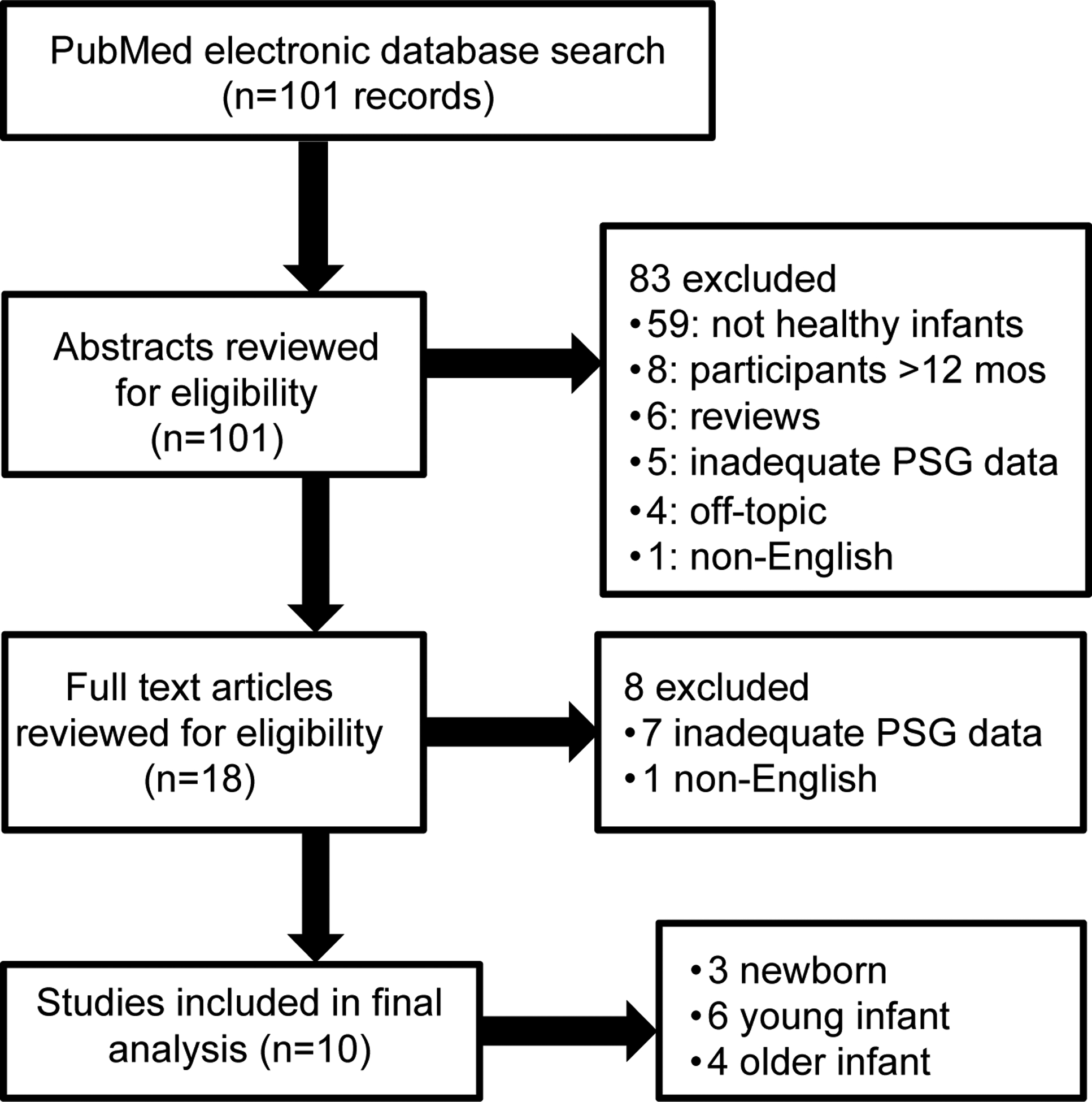
Flowchart of the protocol for article selection.
Due to the high level of heterogeneity in all 6 sleep respiratory indices considered (AHI, OAHI, MAI, OHI, CAI and OAI), we split the studies into three age groups by the age of the participants into: newborns (first month of life), young infants (2–6 months old) and older infants (6–12 months old). Nevertheless, this change did not result in notable decrease in heterogeneity which remained high.
The overall AHI was estimated to be 6.54 (95% CI [3.33, 9.75], Figure 4).
Figure 4.
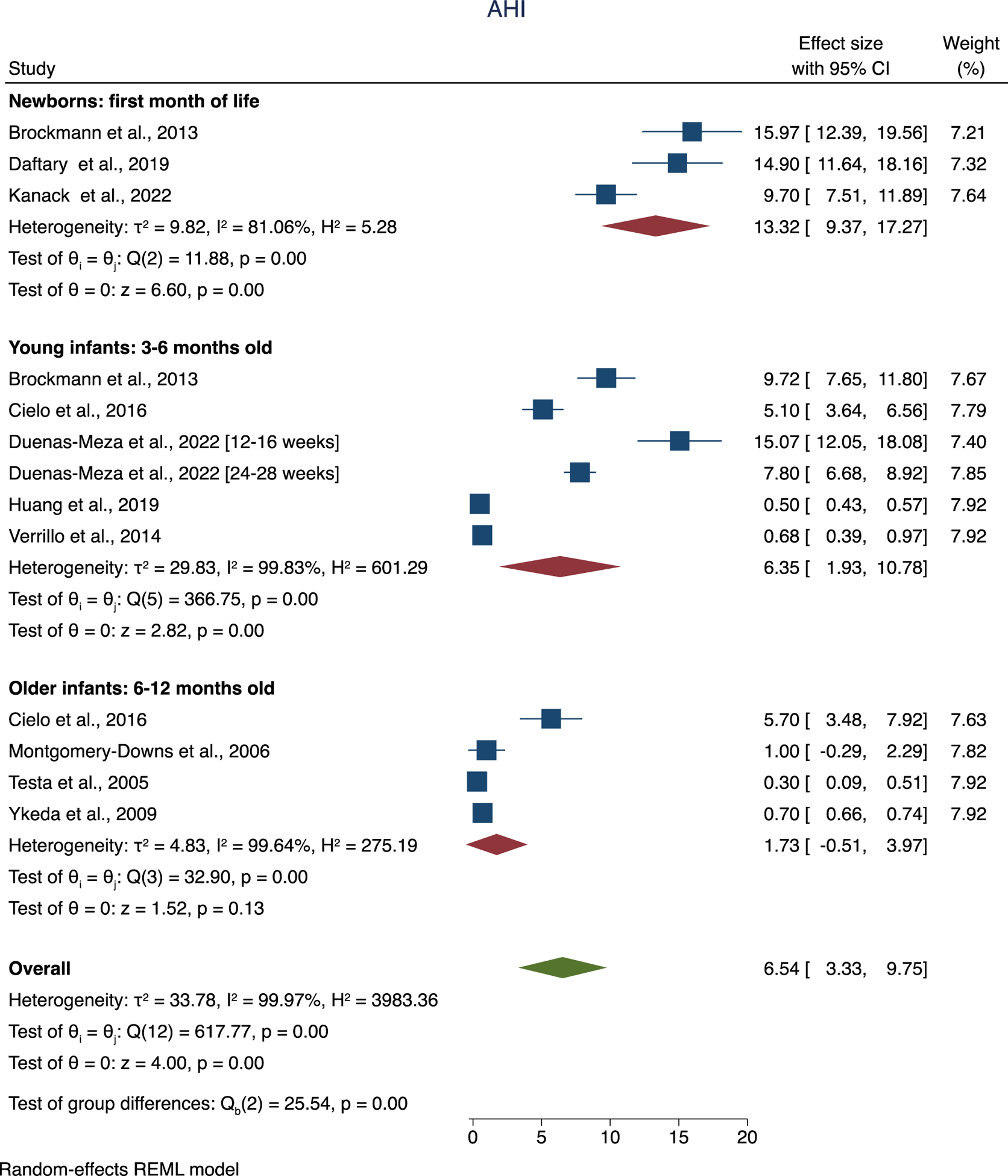
Forest plot of overall and per group mean of AHI.
Furthermore, using a random-effects meta regression, there was a significant decrease in AHI for both young (b: −7.13; 95% CI [−13.36, −0.89]; P=0.025) and old infants (b: - 11.59; 95% CI [−18.23, −4.85]; P=0.001) in comparison to the newborn group. There was not a significant difference in AHI between young and old infants. Overall OAHI was estimated to be 3.53 (95% CI [1.95, 5.11], Supplemental Figure 1).
Of note, OAHI was less frequently reported than AHI, with only one study that reported this index in older infants. There were no differences in OAHI between the three age groups. MA index was estimated to be 0.82 (95% CI [0.34, 1.30], Supplemental Figure 2). MA index was less frequently reported than OAHI or AHI, and again no significant differences were found between the age groups. OH index was estimated to be 2.07 (95% CI [0.34, 3.8]; Supplemental Figure 3). The large level of variance of this index resulted in non-physiological lower bound, again indicating a high level of heterogeneity.
CA index was estimated to be 6.59 (95% CI [4.27, 8.91], Supplemental Figure 4).
OA index was estimated to be 1.26 (95% CI: [0.51, 2.02], Supplemental Figure 5).
There were not significant age group-related differences with the available data for OA and CA indices.
There was significant heterogeneity in reported oxyhemoglobin saturation data between studies included for secondary analysis, especially with both cohorts from Duenas-Meza and colleagues, which was conducted at altitude and overall had lower saturation nadir. SpO2 nadir was estimated to be 85.4% (95% CI: [81.7, 89.1], Figure 5).
Figure 5.
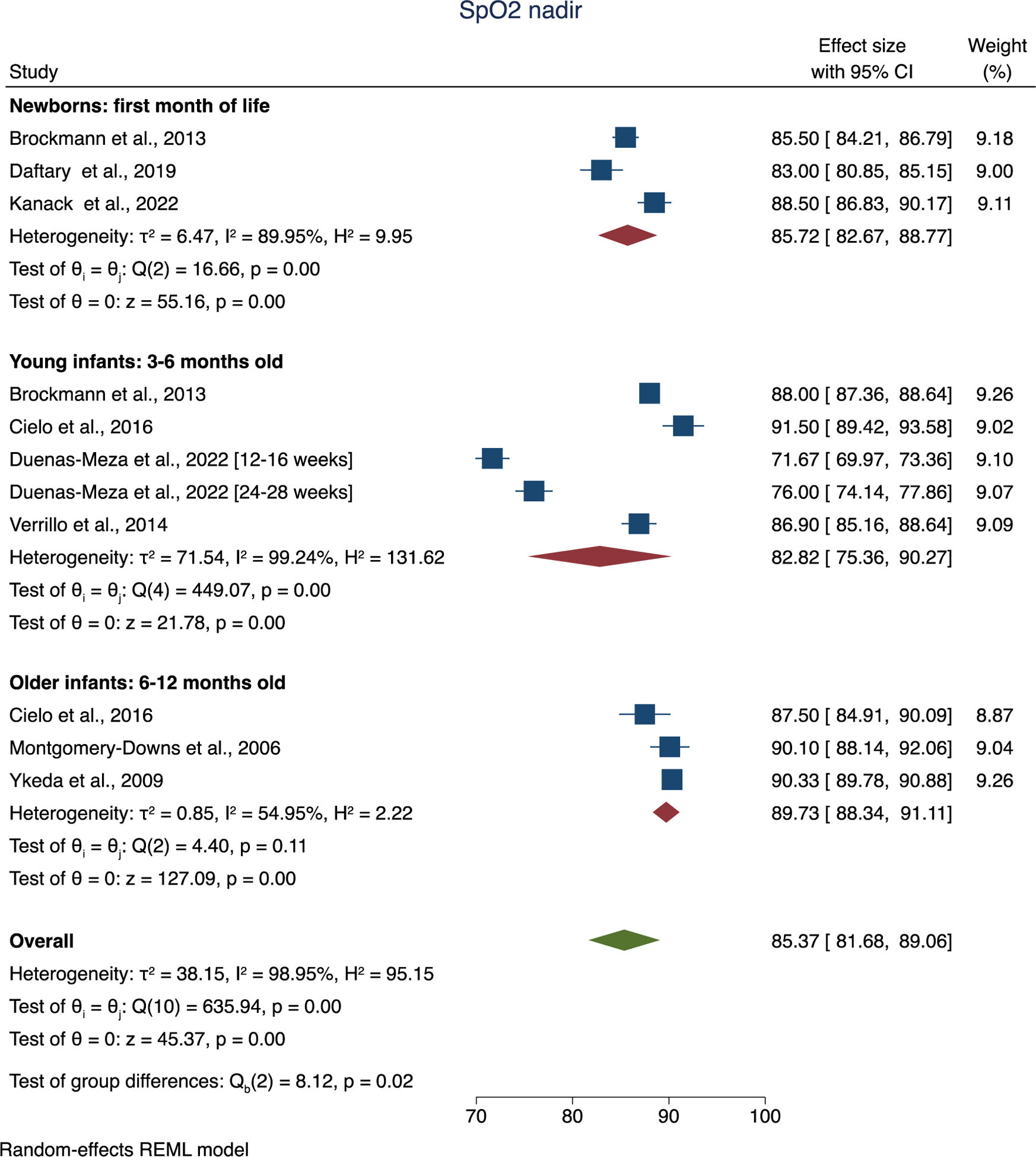
Forest plot of overall and group mean of oxyhemoglobin saturation (SpO2) nadir.
Mean SpO2 was estimated to be 97.6% (95% CI: [97.0, 98.2], Supplemental Figure 6).
Proportion of total sleep time with saturation below 90% was estimated to be 2.4% (95% CI: [0.1, 4.8], Supplemental Figure 7).
There were not significant age group-related differences for any of the saturation indices evaluated.
4. Discussion:
In this observational cohort study, we compared respiratory parameters during sleep as measured by PSG at one month of life to a follow-up at five months old. We found that at one month of age, there was a significant amount of obstructive and central events during sleep in healthy infants. However, in our cohort, at 5 months of age, both obstructive AHI and central apnea index decreased significantly. Importantly, the type of most residual events in healthy infants at 5 months of age changed, with a preponderance of central apneas or obstructive hypopneas and minimal obstructive apneas. Our cohort, which uniquely included longitudinal data using full overnight polysomnography, suggest that at one month of age, an obstructive AHI of greater than 18 events per hour or an obstructive apnea index of greater than 9 events per hour would be considered abnormal. Data from both our primary data and meta-analysis suggest that a central apnea index greater than 9 events per hour would be outside the 95% confidence interval for infants.
Using full in-lab overnight PSG in a racially diverse cohort, this finding supports other studies that included PSG in healthy infants. Our findings at one month of age are consistent with the findings of our meta-analysis, confirming a wide spectrum of both central and obstructive events during sleep in healthy newborns, greater than normative data for older children. Previous studies included in that meta-analysis have largely been limited by cross-sectional design. Data from our cohort found that even in healthy infants with the greatest number of apneic events at one month old, there is significant reduction by five months of age. This finding is similar to the pattern seen by Brockmann and colleagues, which was one of the few previous studies that included longitudinal assessment4. This decrease may be due to improved respiratory mechanics, increased functional residual capacity, more mature ventilatory control, and reduced REM sleep7,11.
Our meta-analysis did find a reduction in overall AHI from newborns to infants older than one month old. Trends of reductions in other indices from younger to older age groups were seen, but were not statistically significant, possibly related in part to overall heterogeneity. The causes of this heterogeneity may be multifactorial, but are likely due to spectrum factors, including but not limited to differences in methodology and within-group age as well as within-sample homogeneity. Nevertheless, estimated mean values for all indices other than obstructive hypopnea index that had non-zero overlapping 95% CI indicating a significant mean value that is different from 0.
In addition to obstructive AHI, data from our cohort suggest that the proportion of obstructive apneas may also be a useful marker of more pathologic OSA. Obstructive apneas represented a relatively low proportion of total events in one-month-old infants, and an even smaller proportion at five months of age. These data support the growing literature that newborn infants with an elevated AHI using established pediatric standards do not necessarily have pathologic obstructive sleep apnea and highlight the need for specific polysomnographic respiratory interpretation for newborns and infants. Accordingly, this could include re-classification of OSA status based on AHI and utilizing repeat polysomnography in this age group.
While the focus of this study was the progression of apneas and hypopneas during sleep in healthy infants, the natural history of OSA in high-risk groups like those with craniofacial conditions and Down syndrome remains poorly understood. In infants with micrognathia and glossoptosis, surgical correction during the newborn period with mandibular distraction osteogenesis is widely accepted as a standard, highly effective therapy demonstrated by improvement or resolution of OSA in many infants18,26. However, OSA will improve in a portion of these patients with growth and conservative therapy such as positive airway pressure, supplemental oxygen, or watchful waiting19,34. In these patients, determining how much of the improvement seen after surgical treatment is due to growth and which patients will improve without surgery is critical in designing treatment algorithms. The natural history of OSA in other high-risk infants, such as those with Down syndrome, is also poorly understood and would benefit from longitudinal studies35,36. Infants with high-risk conditions whose PSG results are only modestly different than healthy controls may benefit from conservative management with re-evaluation rather than surgical intervention.
Using the current American Academy of Sleep Medicine scoring guidelines, respiratory events in children of all ages, including infants, are scored using the same criteria6. However, until recently, studies that aimed to establish normative values for respiratory events on PSG excluded children under a year of age37–39. Apneas are scored if there is a greater than 90% reduction in airflow for the duration of at least two missed breaths, which may be only a few seconds in an infant with a relatively rapid respiratory rate40. Obstructive hypopneas are scored if there is a reduction in airflow of greater than 30% accompanied by snoring, nasal pressure flattening, or paradoxical breathing. However, snoring is not sensitive or specific for OSA in infants and paradoxical breathing during sleep can be normal in this age group, also contributing to the difficulty in distinguishing pathological from physiologic respiratory events during sleep in the youngest patients9,41. It may be helpful to examine these criteria in the context of infants to avoid misinterpreting physiological events as pathological. In addition, the impact of demographic factors, such as sex, prematurity status, race/ethnicity air pollutants, and altitude on respiratory sleep findings should be examined in larger cohorts in this age group. Two studies by the same group studying infants at altitude in Bogota, Colombia have found that there is a higher rate of both central and obstructive respiratory events as well as desaturation during sleep in preterm compared to full-term infants28,42, but direct comparisons have not been made at sea level.
Strengths of our study include longitudinal follow-up using full, in-lab overnight PSG, the gold standard for OSA assessment and a cohort that was much more diverse than previous studies. However, limited sample size that precluded sub-analyses based on race/ethnicity and sex. Since participants were carefully screened to avoid any parental concern for OSA and evaluated for any predisposing condition, we believe that this is truly a reflection of normal evolution of infant anatomy and physiology. Rapid respiratory rate in infants, often precluding an end-tidal plateau, may result in artifactually low end-tidal CO2 measurements, possibly reducing the prevalence of detected hypoventilation during sleep. Participants in this study were only evaluated through the first six months of life, and future longitudinal studies should re-evaluate later in infancy and into childhood. Future studies should evaluate whether infants with greater AHI during the newborn period are at increased risk later in childhood. In addition, future studies should also consider surrogate evaluation of obstructive sleep apnea such as exhaled nitric oxide and exhaled breath condensate, which have shown promise in older patients43,44.
In summary, healthy infants experience more obstructive and central respiratory events during sleep with a wider range of normal than older children, and there are fewer of these after one month of age. These findings support the existing literature regarding normative PSG data in the first month of life and demonstrate that the obstructive AHI can be used effectively to distinguish between healthy infants and those with OSA. These findings should be confirmed with additional infant groups that are high-risk for OSA and evaluate the trajectory and impact of OSA in those infants to improve treatment strategies. Meta-analysis of PSG studies of healthy infants confirms these overall higher obstructive and central indices. While a lower overall AHI was seen in infants greater than one month of age than newborns, there was significant heterogeneity in PSG indices in the mostly small studies available in the literature.
Supplementary Material
Acknowledgments:
The authors thank the technologists and staff at the Children’s Hospital of Philadelphia sleep laboratory who helped conduct this study, especially Michelle Ward. We would like to acknowledge the efforts of Ms. Caroline Melly in assisting with subject recruitment. The authors are grateful to the infants and their families for their enthusiastic participation in this study.
Funding:
This work was supported by National Institutes of Health [grant number K23 HL135346 (CC) and HL155934-01A1 (SS)].
Abbreviations:
- AHI
apnea hypopnea index
- CAI
central apnea index
- MAI
mixed apnea index
- OAHI
obstructive apnea hypopnea index
- OAI
obstructive apnea index
- OHI
obstructive hypopnea index
- OSA
obstructive sleep apnea
- PSG
Polysomnogram
- REM
Rapid eye movement
Footnotes
Disclosure statement:
Financial disclosure: none. Non-financial disclosure: none.
References
- 1.Marcus CL, Brooks LJ, Draper KA, Gozal D. Clinical practice guideline: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3):1–9. [DOI] [PubMed] [Google Scholar]
- 2.Kato I, Franco P, Groswasser J, Kelmanson I, Togari H, Kahn A. Frequency of obstructive and mixed sleep apneas in 1,023 infants. Sleep. 2000;23(4):487–492. [PubMed] [Google Scholar]
- 3.Daftary AS, Jalou HE, Shively L, Slaven JE, Davis SD. Polysomnography Reference Values in Healthy Newborns. J Clin Sleep Med. 2019;15(3):437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brockmann PE, Poets A, Poets CF. Reference values for respiratory events in overnight polygraphy from infants aged 1 and 3months. Sleep Med. 2013;14(12):1323–1327. [DOI] [PubMed] [Google Scholar]
- 5.Redline S, Tishler PV, Schluchter M, Aylor J, Clark K, Graham G. Risk factors for sleep-disordered breathing in children. Associations with obesity, race, and respiratory problems. Am J Respir Crit Care Med. 1999;159(5 Pt 1):1527–1532. [DOI] [PubMed] [Google Scholar]
- 6.Berry RB, Quan SF, Abreu AR. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications. 2.6 ed. Darien, IL: American Academy of Sleep Medicine; 2020. [Google Scholar]
- 7.Isono S, Tanaka A, Ishikawa T, Nishino T. Developmental changes in collapsibility of the passive pharynx during infancy. Am J Respir Crit Care Med. 2000;162(3 Pt 1):832–836. [DOI] [PubMed] [Google Scholar]
- 8.Marcus CL, Fernandes Do Prado LB, Lutz J, et al. Developmental changes in upper airway dynamics. Journal of applied physiology. 2004;97(1):98–108. [DOI] [PubMed] [Google Scholar]
- 9.Gaultier C, Praud JP, Canet E, Delaperche MF, D’Allest AM. Paradoxical inward rib cage motion during rapid eye movement sleep in infants and young children. J Dev Physiol. 1987;9(5):391–397. [PubMed] [Google Scholar]
- 10.Rebuffat E, Groswasser J, Kelmanson I, Sottiaux M, Kahn A. Polygraphic evaluation of night-to-night variability in sleep characteristics and apneas in infants. Sleep. 1994;17(4):329–332. [PubMed] [Google Scholar]
- 11.Katz ES, Mitchell RB, D’Ambrosio CM. Obstructive sleep apnea in infants. Am J Respir Crit Care Med. 2012;185(8):805–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goffinski A, Stanley MA, Shepherd N, et al. Obstructive sleep apnea in young infants with Down syndrome evaluated in a Down syndrome specialty clinic. Am J Med Genet A. 2015;167A(2):324–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Logjes RJH, MacLean JE, de Cort NW, et al. Objective measurements for upper airway obstruction in infants with Robin sequence: what are we measuring? A systematic review. J Clin Sleep Med. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen M, Hamilton J, Narang I. Clinically important age-related differences in sleep related disordered breathing in infants and children with Prader-Willi Syndrome. PLoS One. 2014;9(6):e101012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verkest V, Verhulst S, Van Hoorenbeeck K, Vanderveken O, Saldien V, Boudewyns A. Prevalence of obstructive sleep apnea in children with laryngomalacia and value of polysomnography in treatment decisions. Int J Pediatr Otorhinolaryngol. 2020;137:110255. [DOI] [PubMed] [Google Scholar]
- 16.Cielo CM, Duffy KA, Taylor JA, Marcus CL, Kalish JM. Obstructive Sleep Apnea in Children With Beckwith-Wiedemann Syndrome. J Clin Sleep Med. 2019;15(3):375–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen JL, Cielo CM, Kupa J, et al. The Utility of Early Tongue Reduction Surgery for Macroglossia in Beckwith-Wiedemann Syndrome. Plast Reconstr Surg. 2020;145(4):803e–813e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldstein JA, Chung C, Paliga JT, et al. Mandibular distraction osteogenesis for the treatment of neonatal tongue-based airway obstruction. J Craniofac Surg. 2015;26(3):634–641. [DOI] [PubMed] [Google Scholar]
- 19.Cielo CM, Hernandez P, Ciampaglia AM, Xanthopoulos MS, Beck SE, Tapia IE. Positive airway pressure for the treatment of obstructive sleep apnea syndrome in infants. Chest. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Lieshout MJ, Joosten KF, Mathijssen IM, et al. Robin sequence: A European survey on current practice patterns. Journal of cranio-maxillo-facial surgery : official publication of the European Association for Cranio-Maxillo-Facial Surgery. 2015;43(8):1626–1631. [DOI] [PubMed] [Google Scholar]
- 21.Fan KL, Mandelbaum M, Buro J, et al. Current Trends in Surgical Airway Management of Neonates with Robin Sequence. Plast Reconstr Surg Glob Open. 2018;6(11):e1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. [DOI] [PubMed] [Google Scholar]
- 23.Berkey CS, Hoaglin DC, Mosteller F, Colditz GA. A random-effects regression model for meta-analysis. Stat Med. 1995;14(4):395–411. [DOI] [PubMed] [Google Scholar]
- 24.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
- 26.Cielo CM, Taylor JA, Vossough A, et al. Evolution of Obstructive Sleep Apnea in Infants with Cleft Palate and Micrognathia. J Clin Sleep Med. 2016;12(7):979–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanack MD, Nakra N, Ahmad I, Vyas RM. Normal Neonatal Sleep Defined: Refining Patient Selection and Interpreting Sleep Outcomes for Mandibular Distraction. Plastic and reconstructive surgery Global open. 2022;10(1):e4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duenas-Meza E, Escamilla-Gil MI, Bazurto-Zapata MA, et al. Intermittent hypoxia and respiratory patterns during sleep of preterm infants aged 3 to 18 months residing at high altitude. Sleep. 2022;45(1). [DOI] [PubMed] [Google Scholar]
- 29.Verrillo E, Bruni O, Pavone M, et al. Sleep architecture in infants with spinal muscular atrophy type 1. Sleep Med. 2014;15(10):1246–1250. [DOI] [PubMed] [Google Scholar]
- 30.Huang YS, Hsu JF, Paiva T, Chin WC, Chen IC, Guilleminault C. Sleep-disordered breathing, craniofacial development, and neurodevelopment in premature infants: a 2-year follow-up study. Sleep medicine. 2019;60:20–25. [DOI] [PubMed] [Google Scholar]
- 31.Montgomery-Downs HE, Gozal D. Snore-associated sleep fragmentation in infancy: mental development effects and contribution of secondhand cigarette smoke exposure. Pediatrics. 2006;117(3):e496–502. [DOI] [PubMed] [Google Scholar]
- 32.Testa MB, Pavone M, Bertini E, Petrone A, Pagani M, Cutrera R. Sleep-disordered breathing in spinal muscular atrophy types 1 and 2. American journal of physical medicine & rehabilitation. 2005;84(9):666–670. [DOI] [PubMed] [Google Scholar]
- 33.Ykeda DS, Lorenzi-Filho G, Lopes AA, Alves RS. Sleep in infants with congenital heart disease. Clinics (Sao Paulo, Brazil). 2009;64(12):1205–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ehsan Z, Kurian C, Weaver KN, et al. Longitudinal Sleep Outcomes in Neonates With Pierre Robin Sequence Treated Conservatively. J Clin Sleep Med. 2019;15(3):477–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waters KA, Castro C, Chawla J. The spectrum of obstructive sleep apnea in infants and children with Down Syndrome. Int J Pediatr Otorhinolaryngol. 2020;129:109763. [DOI] [PubMed] [Google Scholar]
- 36.Hill CM, Evans HJ, Elphick H, et al. Prevalence and predictors of obstructive sleep apnoea in young children with Down syndrome. Sleep Med. 2016;27–28:99–106. [DOI] [PubMed] [Google Scholar]
- 37.Marcus CL, Omlin KJ, Basinki DJ, et al. Normal polysomnographic values for children and adolescents. Am Rev Respir Dis. 1992;146(5 Pt 1):1235–1239. [DOI] [PubMed] [Google Scholar]
- 38.Uliel S, Tauman R, Greenfeld M, Sivan Y. Normal polysomnographic respiratory values in children and adolescents. Chest. 2004;125(3):872–878. [DOI] [PubMed] [Google Scholar]
- 39.Verhulst SL, Schrauwen N, Haentjens D, Van Gaal L, De Backer WA, Desager KN. Reference values for sleep-related respiratory variables in asymptomatic European children and adolescents. Pediatr Pulmonol. 2007;42(2):159–167. [DOI] [PubMed] [Google Scholar]
- 40.Schafer T, Schafer D, Schlafke ME. Breathing, transcutaneous blood gases, and CO2 response in SIDS siblings and control infants during sleep. Journal of applied physiology. 1993;74(1):88–102. [DOI] [PubMed] [Google Scholar]
- 41.Kahn A, Groswasser J, Sottiaux M, et al. Clinical symptoms associated with brief obstructive sleep apnea in normal infants. Sleep. 1993;16(5):409–413. [DOI] [PubMed] [Google Scholar]
- 42.Duenas-Meza E, Bazurto-Zapata MA, Gozal D, Gonzalez-Garcia M, Duran-Cantolla J, Torres-Duque CA. Overnight Polysomnographic Characteristics and Oxygen Saturation of Healthy Infants, 1 to 18 Months of Age, Born and Residing At High Altitude (2,640 Meters). Chest. 2015;148(1):120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goldbart AD, Krishna J, Li RC, Serpero LD, Gozal D. Inflammatory mediators in exhaled breath condensate of children with obstructive sleep apnea syndrome. Chest. 2006;130(1):143–148. [DOI] [PubMed] [Google Scholar]
- 44.Zhang D, Luo J, Qiao Y, Xiao Y, Huang R, Zhong X. Measurement of exhaled nitric oxide concentration in patients with obstructive sleep apnea: A meta-analysis. Medicine (Baltimore). 2017;96(12):e6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


